Sunmark sinus
McKesson Sinus Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient (in each caplet)
- Purpose
- Sunmark sinus Uses
- Warnings
- Directions
- Inactive ingredients
- Questions or comments?
- Principal Display Panel
FULL PRESCRIBING INFORMATION
Active ingredient (in each caplet)
Acetaminophen 325 mg
Phenylephrine HCl 5 mg
Purpose
Pain reliever
Nasal decongestant
Sunmark sinus Uses
- for the temporary relief of:
- headache
- sinus congestion and pressure
- nasal congestion
- minor aches and pains
- helps decongest sinus openings and passages
- promotes sinus drainage
- helps clear nasal passages
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 12 caplets in 24 hours, which is the maximum daily amount for this product
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- liver disease
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- trouble urinating due to an enlarged prostate gland
Ask a doctor or pharmacist before use if you are
taking the blood thinning drug warfarin
When using this product
do not use more than directed
Stop use and ask a doctor if
- you get nervous, dizzy or sleepless
- pain or nasal congestion gets worse or lasts more than 7 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present
- new symptoms occur
These could be signs of a serious condition.
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222) Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Directions
- do not take more than directed (see Liver warning)
| adults and children 12 years and over |
|
| children under 12 years |
do not use this adult product in children under 12 years of age |
Inactive ingredients
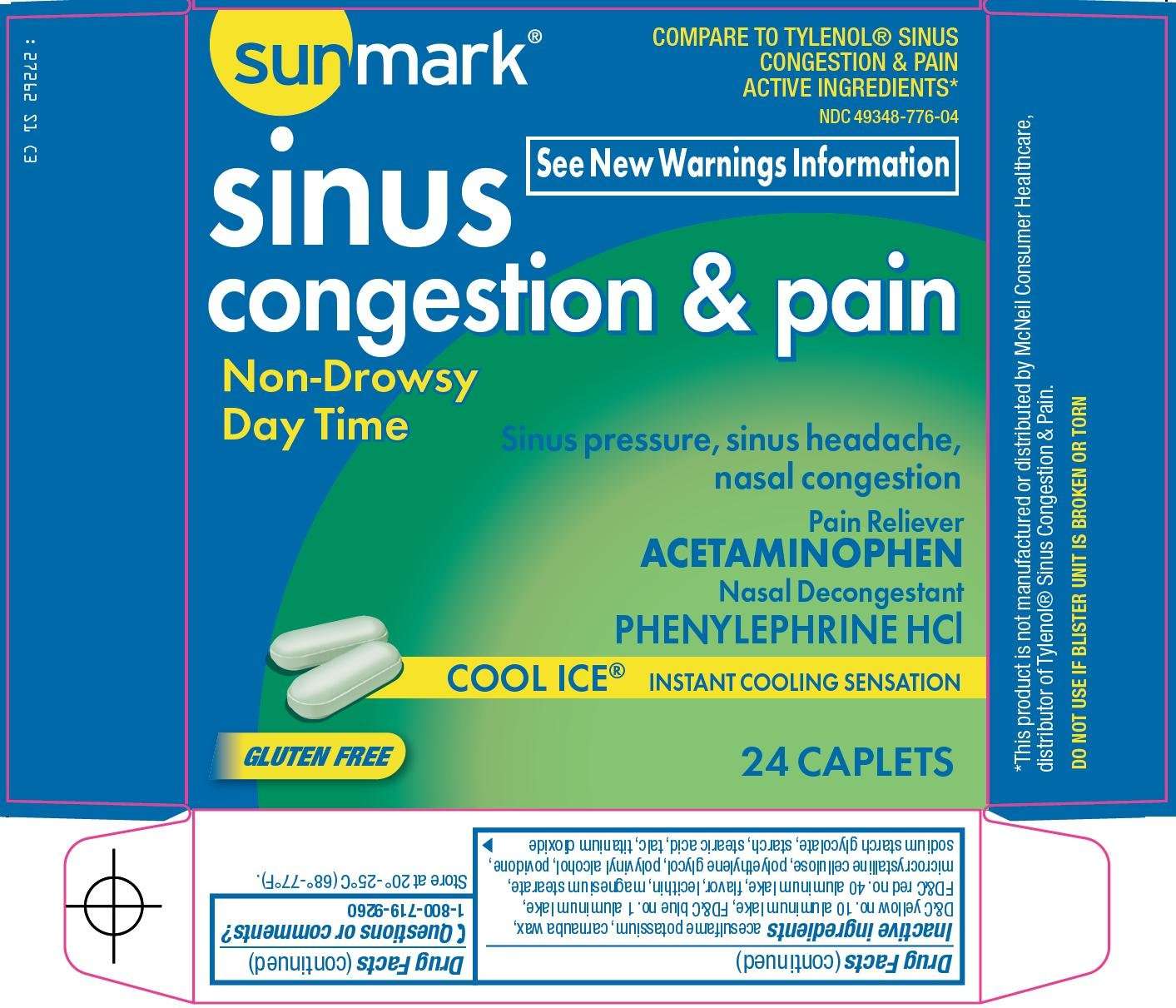
acesulfame potassium, carnauba wax, D&C yellow no. 10 aluminum lake, FD&C blue no. 1 aluminum lake, FD&C red no. 40 aluminum lake, flavor, lecithin, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, povidone, sodium starch glycolate, starch, stearic acid, talc, titanium dioxide
Questions or comments?
1-800-719-9260
Principal Display Panel
COMPARE TO TYLENOL® SINUS CONGESTIN & PAIN ACTIVE INGREDIENTS
See New Warnings Information
sinus
congestion & pain
Non-Drowsy
Day Time
Sinus pressure, sinus headache, nasal congestion
Pain Reliever
ACETAMINOPHEN
Nasal Decongestant
PHENYLEPHRINE HCl
COOL ICE®
INSTANT COOLING SENSATION
GLUTEN FREE
Sunmark sinusAcetaminophen, Phenylephrine HCl TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||