Sunmark Nasal
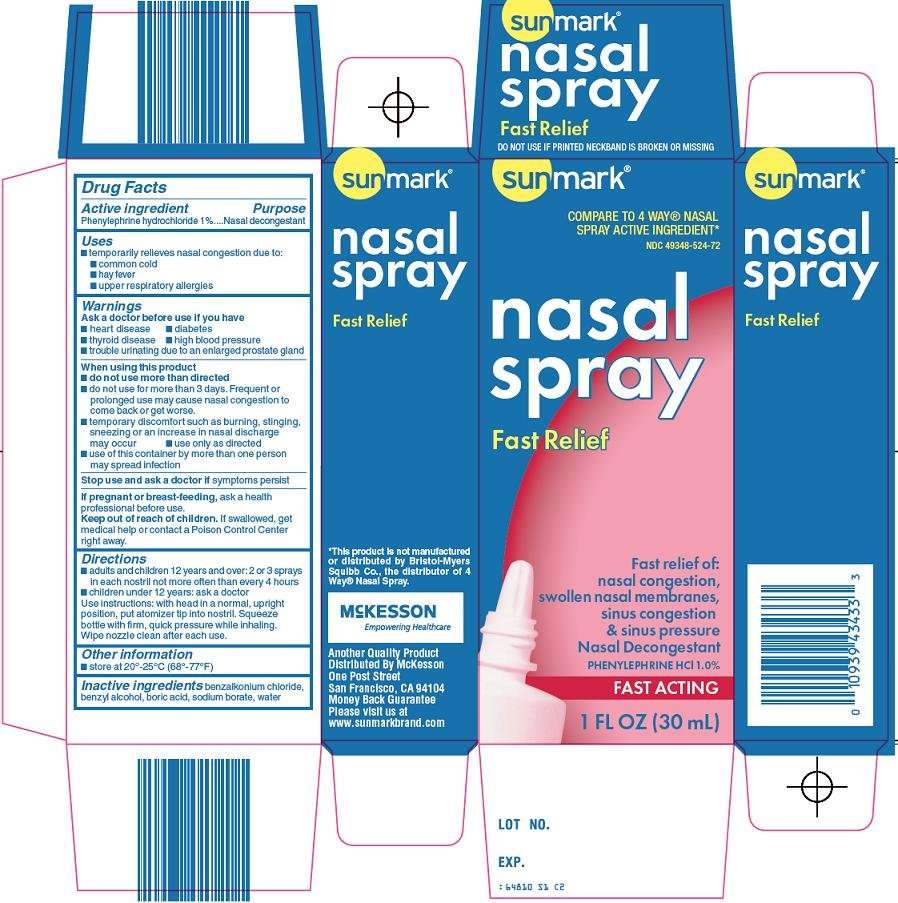
McKesson Nasal Spray Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- Sunmark Nasal Uses
- Warnings
- Directions
- Sunmark Nasal Other information
- Inactive ingredients
- Principal Display Panel
FULL PRESCRIBING INFORMATION
Active ingredient
Phenylephrine hydrochloride 1%
Purpose
Nasal decongestant
Sunmark Nasal Uses
- temporarily relieves nasal congestion due to:
- common cold
- hay fever
- upper respiratory allergies
Warnings
Ask a doctor before use if you have
- heart disease
- diabetes
- thyroid disease
- high blood pressure
- trouble urinating due to an enlarged prostate gland
When using this product
- do not use more than directed
- do not use for more than 3 days. Frequent or prolonged use may cause nasal congestion to come back or get worse.
- use only as directed
- temporary discomfort such as burning, stinging, sneezing or an increase in nasal discharge may occur
- use of this container by more than one person may spread infection
Stop use and ask a doctor if
symptoms persist
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- adults and children 12 years and over: 2 or 3 sprays in each nostril not more often than every 4 hours
- children under 12 years: ask a doctor
Use instructions: with head in a normal, upright position, put atomizer tip into nostril. Squeeze bottle with firm, quick pressure while inhaling. Wipe nozzle clean after each use.
Sunmark Nasal Other information
- store at 20°-25°C (68°-77°F)
Inactive ingredients
benzalkonium chloride, benzyl alcohol, boric acid, sodium borate, water
Principal Display Panel
Compare to 4 Way® Nasal Spray Active Ingredient
Nasal Spray
Fast Relief
Fast Relief of:
Nasal Congestion, Swollen Nasal Membranes, Sinus Congestion & Sinus Pressure
Nasal Decongestant
Phenylephrine HCl 1.0%
Fast Acting
Nasal Spray Carton
Sunmark NasalPhenylephrine hydrochloride SPRAY
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!