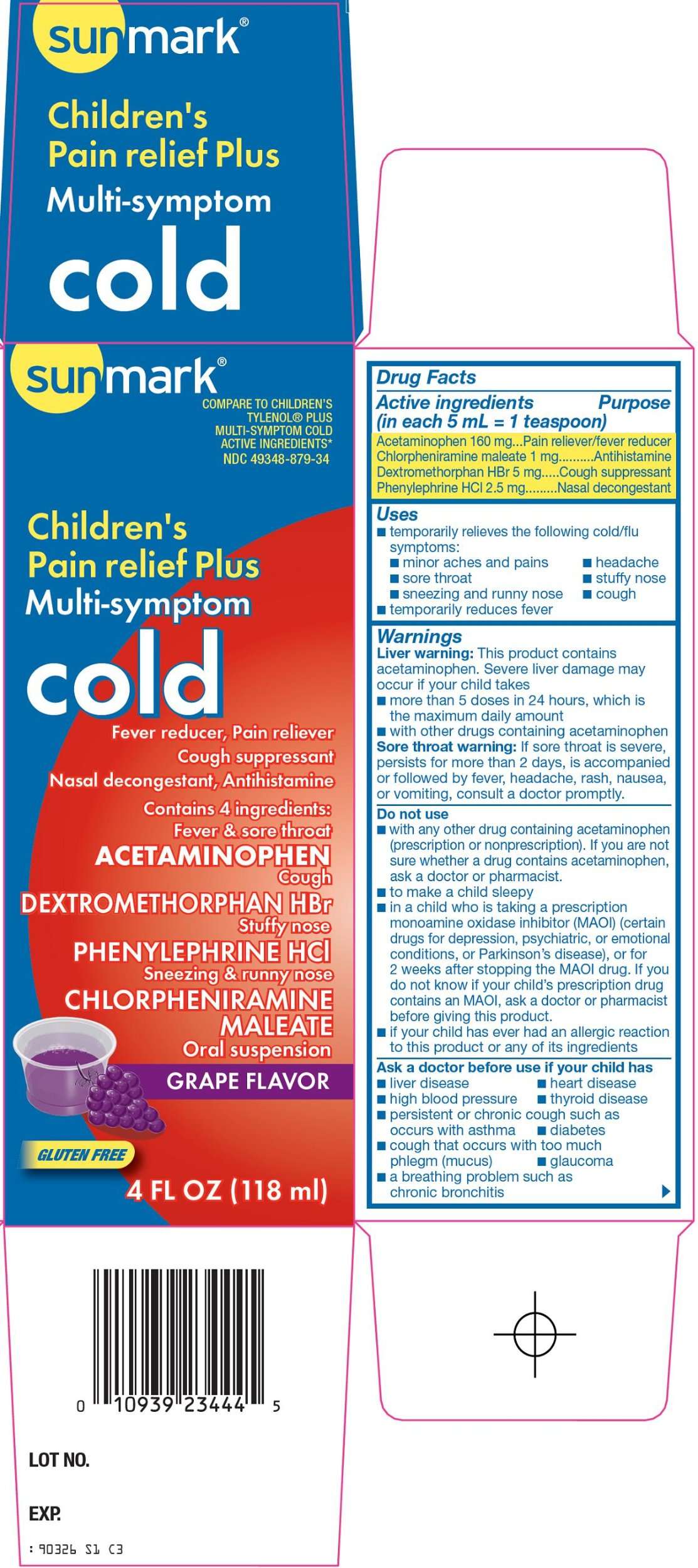
sunmark Cold
McKesson Cold Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredients (in each 5 mL = 1 teaspoon)
- Purpose
- sunmark Cold Uses
- Warnings
- Directions
- sunmark Cold Other information
- Inactive ingredients
- Questions or comments?
- Principal Display Panel
FULL PRESCRIBING INFORMATION
Active ingredients (in each 5 mL = 1 teaspoon)
Acetaminophen 160 mg
Chlorpheniramine maleate 1 mg
Dextromethorphan HBr 5 mg
Phenylephrine HCl 2.5 mg
Purpose
Pain reliever/fever reducer
Antihistamine
Cough suppressant
Nasal decongestant
sunmark Cold Uses
- temporarily relieves the following cold/flu symptoms:
- minor aches and pains
- headache
- sore throat
- cough
- stuffy nose
- sneezing and runny nose
- temporarily reduces fever
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if your child takes
- more than 5 doses in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- to make a child sleepy
- in a child who is taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your child’s prescription drug contains an MAOI, ask a doctor or pharmacist before giving this product.
- if your child has ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if your child has
- liver disease
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- persistent or chronic cough such as occurs with asthma
- cough that occurs with too much phlegm (mucus)
- a breathing problem such as chronic bronchitis
- glaucoma
Ask a doctor or pharmacist before use if your child is
- taking the blood thinning drug warfarin
- taking sedatives or tranquilizers
When using this product
- do not exceed recommended dosage
- excitability may occur, especially in children
- may cause marked drowsiness
- sedatives and tranquilizers may increase drowsiness
Stop use and ask a doctor if
- nervousness, dizziness, or sleeplessness occur
- pain, nasal congestion or cough gets worse or lasts more than 5 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present
- new symptoms occur
- cough comes back or occurs with rash or headache that lasts
These could be signs of a serious condition.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222) Quick medical attention is critical even if you do not notice any signs or symptoms.
Directions
- this product does not contain directions or complete warnings for adult use
- do not give more than directed (see Liver warning)
- shake well before using
- find right dose on chart below. If possible, use weight to dose; otherwise, use age.
- use only enclosed dosing cup designed for use with this product. Do not use any other dosing device.
- if needed, repeat dose every 4-6 hours while symptoms last
- do not give more than 5 times in 24 hours
- do not give for more than 5 days unless directed by a doctor
| Weight (lb) | Age (yr) | Dose (tsp or mL) |
| under 36 lbs | under 4 years | do not use |
| 36-47 lbs | 4-5 years | do not use unless directed by a doctor |
| 48-95 lbs | 6-11 years | 2 tsp or 10 mL |
Attention: use only enclosed dosing cup specifically designed for use with this product. Do not use any other dosing device.
sunmark Cold Other information
- each teaspoon contains: sodium 3 mg
- store between 20º-25ºC (68º-77ºF)
- do not use if printed neckband is broken or missing
Inactive ingredients
anhydrous citric acid, calcium sulfate, carrageenan, D&C red #33, edetate disodium, FD&C blue #1, FD&C red #40, flavor, glycerin, polysorbate 80, purified water, sodium benzoate, sorbitol solution, sucrose, tribasic sodium phosphate, xanthan gum
Questions or comments?
1-800-719-9260
Principal Display Panel
COMPARE TO CHILDREN'S TYLENOL® PLUS MULTI-SYMPTOM COLD ACTIVE INGREDIENTS
Children’s
Pain relief Plus
Multi-Symptom
cold
Fever reducer, Pain reliever
Cough suppressant
Nasal decongestant, Antithistamine
Contains 4iIngredients:
Fever & sore throat
ACETAMINOPHEN
Cough
DEXTROMETHORPHAN HBr
Stuffy nose
PHENYLEPHRINE HCl
Sneezing & runny nose
CHLORPHENIRAMINE MALEATE
Oral Suspension
GRAPE FLAVOR
GLUTEN FREE
sunmark ColdAcetaminophen, Chlorpheniramine Maleate, Dextromethorphan HBr, Phenylephrine HCl SUSPENSION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||