Stool Softener
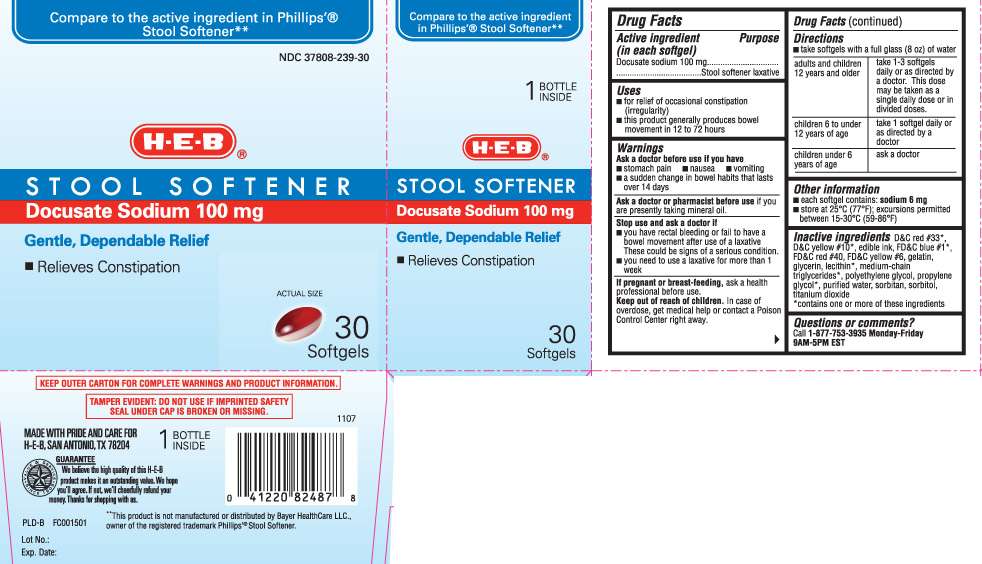
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient (in each softgel)
- Purpose
- Stool Softener Uses
- Warnings
- Directions
- Stool Softener Other information
- Inactive ingredients
- Questions or comments?
- Principal Display Panel
- Product Labeling
FULL PRESCRIBING INFORMATION
Active ingredient (in each softgel)
Docusate sodium 100 mg
Purpose
Stool softener laxative
Stool Softener Uses
- for relief of occasional constipation (irregularity)
- this product generally produces bowel movement in 12 to 72 hours
Warnings
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- a sudden change in bowel habits that lasts over 14 days
Ask a doctor or pharmacist before use
if you are presently taking mineral oil.
Stop use and ask a doctor if
- you have rectal bleeding or fail to have a bowel movement after use of a laxative. These could be signs of a serious condition.
- you need to use a laxative for more than 1 week
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- take softgels with a full glass (8 oz) of water
| adults and children 12 years and older | take 1-3 softgels daily or as directed by a doctor. This dose may be taken as a single daily dose or in divided doses. |
| children 6 to under 12 years of age | take 1 softgel daily or as directed by a doctor |
| children under 6 years of age | ask a doctor |
Stool Softener Other information
- each softgel contains: sodium 6 mg
- store at 25ºC (77ºF); excursions permitted between 15-30ºC (59-86ºF)
Inactive ingredients
D&C red #33*, D&C yellow #10*, edible ink, FD&C blue #1*, FD&C red #40, FD&C yellow #6, gelatin, glycerin, lecithin*, medium-chain triglycerides*, polyethylene glycol, propylene glycol*, purified water, sorbitan, sorbitol, titanium dioxide
*contains one or more of these ingredients
Questions or comments?
Call 1-877-753-3935 Monday-Friday 9AM-5PM EST
Principal Display Panel
Compare to the active ingredient in Phillips'® Stool Softener**
STOOL SOFTENER
Docusate Sodium 100 mg
Gentle, Dependable Relief
• Relieves Constipation
Softgels
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING.
MADE WITH PRIDE AND CARE FOR H-E-B, SAN ANTONIO, TX 78204
**This product is not manufactured or distributed by Bayer HealthCare LLC, owner of the registered trademark Phillips'® Stool Softener.
Product Labeling
Stool SoftenerDOCUSATE SODIUM CAPSULE, LIQUID FILLED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||