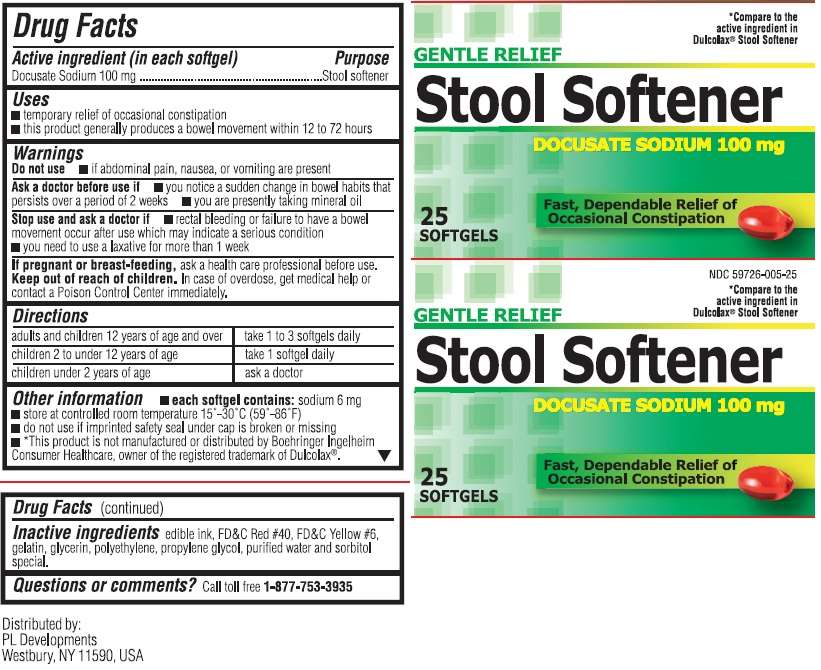
Stool Softener
P & L Development of New York Corporation
Gentle Relief Stool Softener
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredients (in each softgel)
- Stool Softener Uses
- Warnings - Do not use
- Directions
- Stool Softener Other information
- Inactive Ingredients
- Questions or comments?
- Product Labeling
FULL PRESCRIBING INFORMATION
Active Ingredients (in each softgel)
Docusate Sodium 100mg
Purpose
Stool softener
Stool Softener Uses
- temporary relief of occasional constipation.
- This product generally produces a bowel movement within 12 to72 hours.
Warnings - Do not use
Ask a doctor before use if
- you notice a sudden change in bowel habits that persists over a period of 2 weeks.
- you are presently taking mineral oil
Stop use and ask a doctor if
- rectal bleeding or failure to have bowel movement occur after use which may indicate a serious condition
- you need to use a laxative for more than 1 week.
If pregnant or breastfeeding
ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center immediately.
Directions
-
adults and children over 12 years of age: take 1 to 3 softgels daily
-
children 2 to under 12 years of age: take 1 softgel daily
-
children under 2 years of age: ask a doctor
Stool Softener Other information
- each softgel contains: sodium 6mg
- store at controlled room temperature 15 - 30 degrees C (59-86 degrees F)
- do not use if imprinted safety seal under cap is broken or missing.
- *This product is not manufactured or distributed by Boehringer Ingelheim Consumer Healthcare, owner of the registered trademark Dulcolax.
Inactive Ingredients
edible ink, FDandC Red #40, FDandC Yellow #6, gelatin, glycerin, polyethylene, propylene glycol, purified water and sorbitol special.
Questions or comments?
call toll free: 1-877-753-3935
Comapre to the active ingredient in Dulcolax Stool Softener NDC: 59726-005-25
Gentle Relief
Stool Softener
Docusate Sodium 100mg
Fast, Dependable Relief of Occasional Constipation
25 softgels
Distributed by PL Development
Westbury, NY 11590 USA
Product Labeling

Stool SoftenerDOCUSATE SODIUM CAPSULE, LIQUID FILLED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||