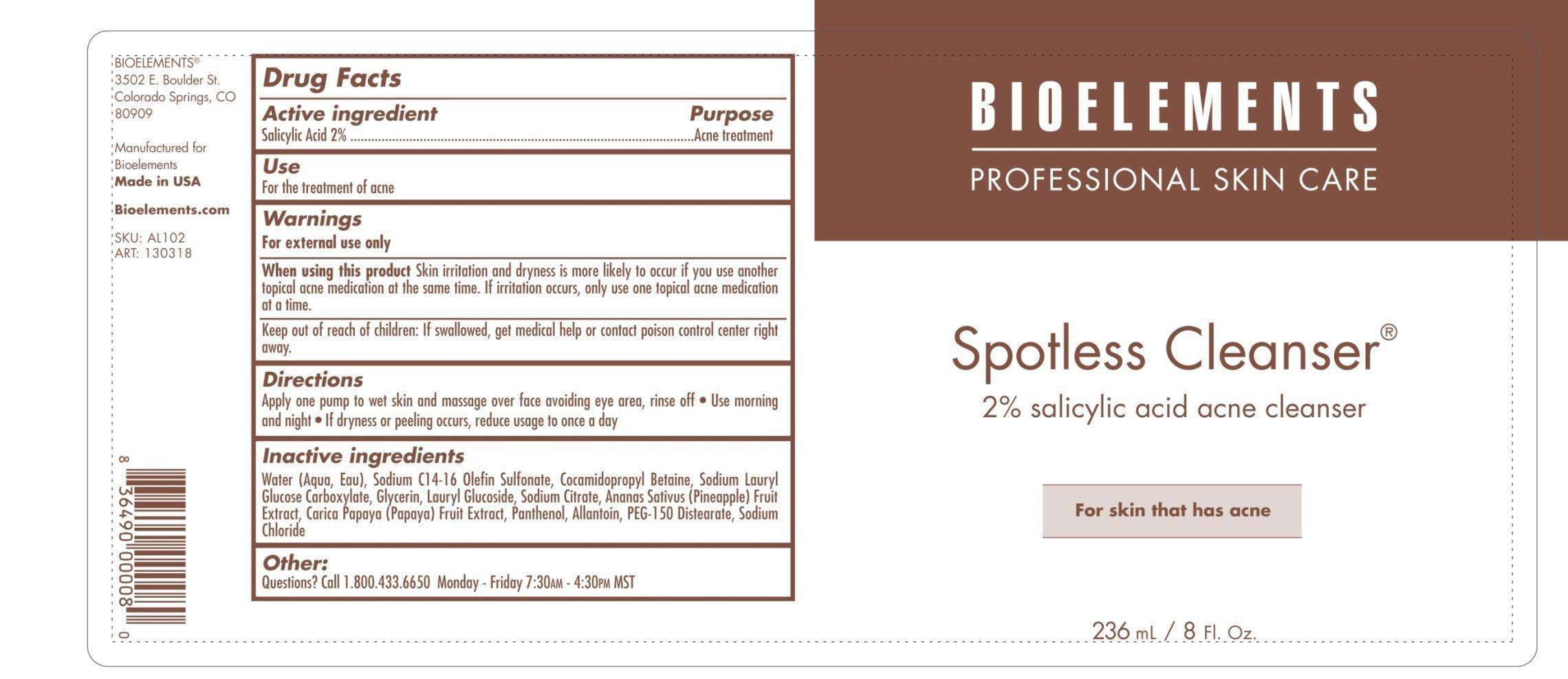
Spotless Cleanser
Bioelements, Inc.
Bioelements, Inc.
Spotless Cleanser
FULL PRESCRIBING INFORMATION
Active ingredient
Active Ingredient Purpose
Salicylic Acid 2% Acne treatment
Purpose
Uses
for the treatment of acne
Keep out of reach of children. If swallowed, get medical help or contact Poison Control Center right away.
Uses
When using this product: Skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs only use one topical acne medication at a time.
Warnings
For external use only
Directions
Apply one pump to wet skin and massage over face avoiding eye area, rinse off.
Use morning and night
If dryness or peeling occurs, reduce usage to once a day.
Inactive ingredients
Water (Aqua, Eau), Sodium C14-16 Olefin Sulfonate, Cocamidopropyl Betaine, Sodium Lauryl Glucose Carboxylate, Glycerin, Lauryl Glucoside, Sodium Citrate, Ananas Sativus (Pineapple) Fruit Extract, Carica Papaya (Papaya) Fruit Extract, Panthenol, Allantoin, PEG-150 Distearate, Sodium Chloride.


Bioelements
Acne Clearing System
Spotless Cleanser
2% salicylic acid acne cleanser
Cleanses deep to clear and heal acne
Spotless CleanserSalicylic Acid CREAM
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||