SPOT REMOVER ACNE TREATMENT PADS
ORIGINS NATURAL RESOURCES INC.
SPOT REMOVER™ ACNE TREATMENT PADS
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- SPOT REMOVER ACNE TREATMENT PADS Uses
- Warnings
- Directions
- Inactive ingredients
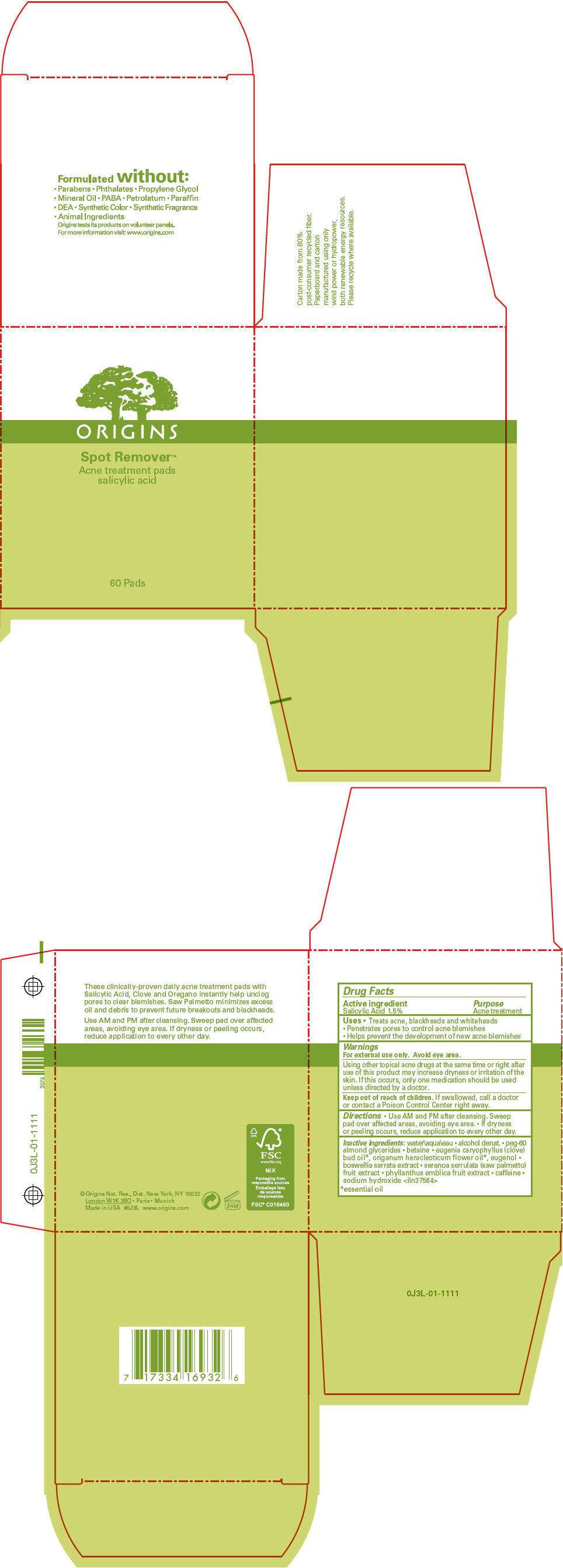
- PRINCIPAL DISPLAY PANEL - 60 Pad Jar Carton
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient
Salicylic Acid 1.5%
Purpose
Acne treatment
SPOT REMOVER ACNE TREATMENT PADS Uses
- Treats acne, blackheads and whiteheads
- Penetrates pores to control acne blemishes
- Helps prevent the development of new acne blemishes
Warnings
For external use only. Avoid eye area.
Using other topical acne drugs at the same time or right after use of this product may increase dryness or irritation of the skin. If this occurs, only one medication should be used unless directed by a doctor.
Keep out of reach of children. If swallowed, call a doctor or contact a Poison Control Center right away.
Directions
- Use AM and PM after cleansing. Sweep pad over affected areas, avoiding eye area.
- If dryness or peeling occurs, reduce application to every other day.
Inactive ingredients
water\aqua\eau • alcohol denat. • peg-60 almond glycerides • betaine • eugenia caryophyllus (clove) bud oil

PRINCIPAL DISPLAY PANEL - 60 Pad Jar Carton
ORIGINS
Spot Remover™
Acne treatment pads
salicylic acid
60 Pads
SPOT REMOVER ACNE TREATMENT PADSSALICYLIC ACID LIQUID
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||