Spider Sense Spider-Man Pediatric
Spider Sense Spider-Man Pediatric Kit
FULL PRESCRIBING INFORMATION: CONTENTS*
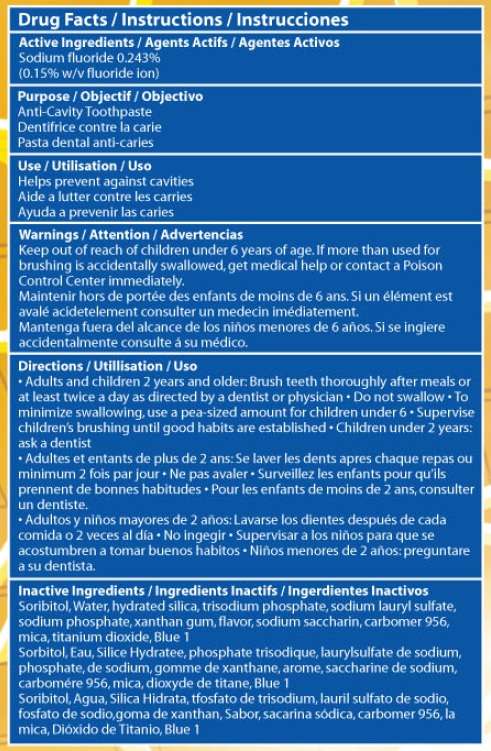
- Active Ingredient
- Purpose
- Spider Sense Spider-Man Pediatric Uses
- Warnings
- Directions
- Inactive Ingredients
- Comments? Questions?
FULL PRESCRIBING INFORMATION
Active Ingredient
Sodium fluoride 0.243% (0.15% w/v fluoride ion)
Purpose
Anticavity toothpaste
Spider Sense Spider-Man Pediatric Uses
Helps prevent against cavities
Warnings
If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center immediately
Keep out of the reach of children under 6 years of age.
Directions
Adults and children 2 years and older: Brush teeth thoroughly after meals or at least twice a day as directed by a dentist or physician. Do not swallow
To minimize swallowing use a pea-sized amount in children under 6.
Supervise children's brushing until good habits are established.
Children under 2 years: Ask a dentist
Inactive Ingredients
Sorbitol, water, hydrated silica, trisodium phosphate, sodium lauryl sulfate, flavor, sodium phosphate, xanthan gum, carbomer 956, sodium saccharin, titanium dioxide, blue no. 1
Comments? Questions?
Toll Free: 1-866-373-7374
comments@drfresh.com
CAUTION/CHOKING HAZARD:



Spider Sense Spider-Man PediatricSODIUM FLUORIDE PASTE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||