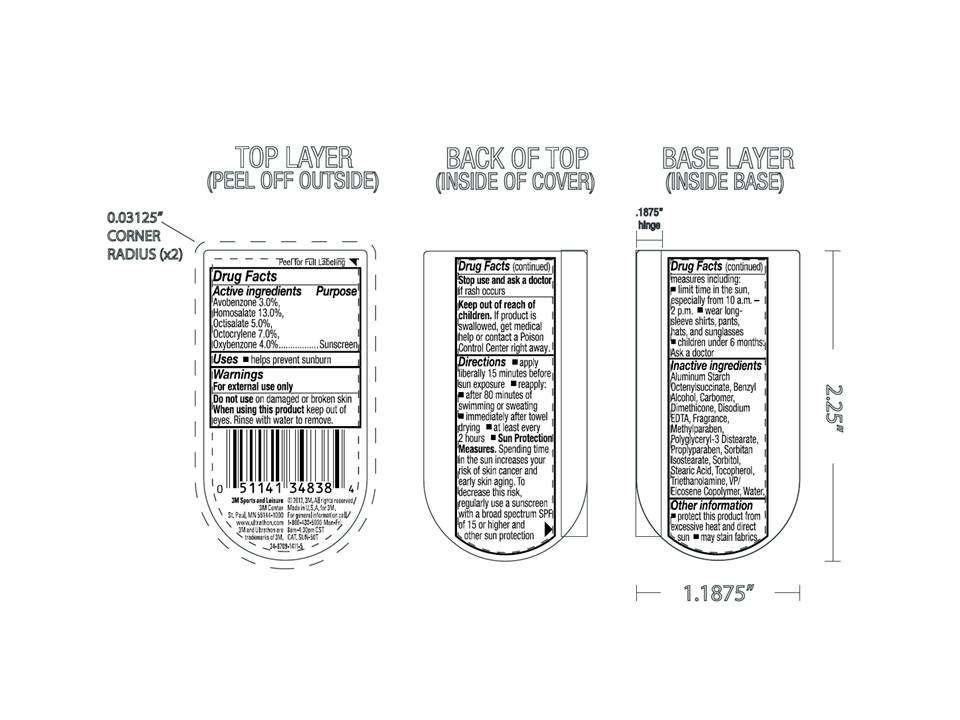
SPF 50 Broad Spectrum
Drug Facts
FULL PRESCRIBING INFORMATION
Active ingredient
Avobenzone 3.0%, Homosalate 13%, Octisalate 5%, Octocrylene 7%, Oxybenzone 4%
Uses
Uses
Helps prevent sunburn.
Warnings
For external use only. Do not use on damaged or broken skin. When using this product: keep out of eyes. Rinse eyes with water to remove. Stop use and ask a doctor if rash occurs.
Directions
Apply liberally 15 minutes before sun exposure. Reapply: after 80 minutes of swimming or sweating, immediately after towel drying, at least every 2 hours. Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease the risk, regularly use a sunscreen with broad spectrum SPF of 15 or higher and other sun protection measures including: limit time in the sun, especially from 10 a.m. - 2 p.m., wear long-sleeved shirts, pants, hats, and sunglasses. Children under 6 months: ask a doctor.
Inactive ingredients
Aluminum Starch Octenylsuccinate, Benzyl Alcohol, Carbomer, Dimethicone, Disodium EDTA, Fragrance, Methylparaben, Polyglyceryl-3 Distearate, Propylparaben, Sorbitan Isostearate, Sorbitol, Stearic Acid, Tocopherol, Triethanolamine, VP/Eicosene Copolymer, Water.
Purpose
Purpose
Sunscreen.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
3M Ultrathon Outdoor Broad Spectrum SPF 50. Water Resistant (80 Minutes).


SPF 50 Broad SpectrumAvobenzone, Homosalate, Octisalate, Octocrylene, Oxybenzone LOTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||