Soothe Hydration
Soothe Hydration Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredients
- Purpose
- Soothe Hydration Uses
- Warnings
- Directions
- Soothe Hydration Other information
- Inactive ingredients
- Questions?
- Package/Label Principal Display Panel
FULL PRESCRIBING INFORMATION
Active ingredients
Povidone (1.25%)
Purpose
Lubricant
Soothe Hydration Uses
- relieves dryness of the eye
- prevents further irritation
Warnings
For external use only.
Do not use
- if solution changes color or becomes cloudy
When using this product
- do not touch tip of container to any surface to avoid contamination
- replace cap after using
Stop use and ask a doctor if you experience
- eye pain
- change in vision
- continued redness or irritation of the eye
- or if condition worsens or lasts more than 72 hours
I f pregnant or breastfeeding, ask a health professional before use.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
-
Soothe Hydration Other information
- Storage: 15° - 25°C (59° - 77°F)
- Use before expiration date marked on the carton or bottle
Inactive ingredients
boric acid, potassium chloride, sodium borate and sodium chloride; preserved with edetate disodium 0.1% and sorbic acid 0.1%
Questions?
Toll Free Product Information or to Report a Serious Side Effect Associated with use of the product Call: 1-800-553-5340
®/TM are trademarks of Bausch & Lomb Incorporated.
Marketed by:
©Bausch & Lomb Incorporated
Rochester, NY 14609
Order No. 622266
DO NOT USE IF IMPRINTED NECKBAND IS NOT INTACT.
Package/Label Principal Display Panel
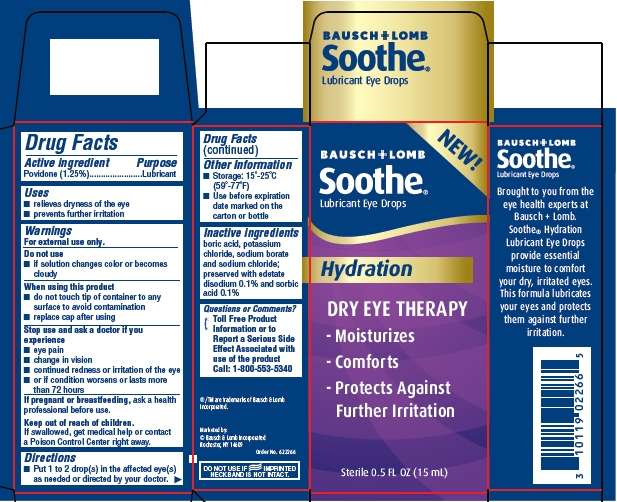
Bausch & Lomb® NEW!
Soothe®
Lubricant Eye Drops
Hydration
DRY EYE THERAPY
Moisturizes
Comforts
Protects Against
Further Irritation
Sterile 0.5 FL OZ (15mL)
Soothe Hydrationpovidone SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||