Sooryehan Pure Whitening Spot Treatment
LG Household and Healthcare, Inc.
LG Household and Healthcare, Inc.
DRUG FACTS
FULL PRESCRIBING INFORMATION
Active ingredient
POLYDATIN 0.21mL/100mL
For external use only
Keep out of reach of children. Is swallowed, get medical help or contact a Poison Control Center right away.
Keep out of eyes. Rinse with water to remove.
Stop use if a rash or irritation develops and lasts.
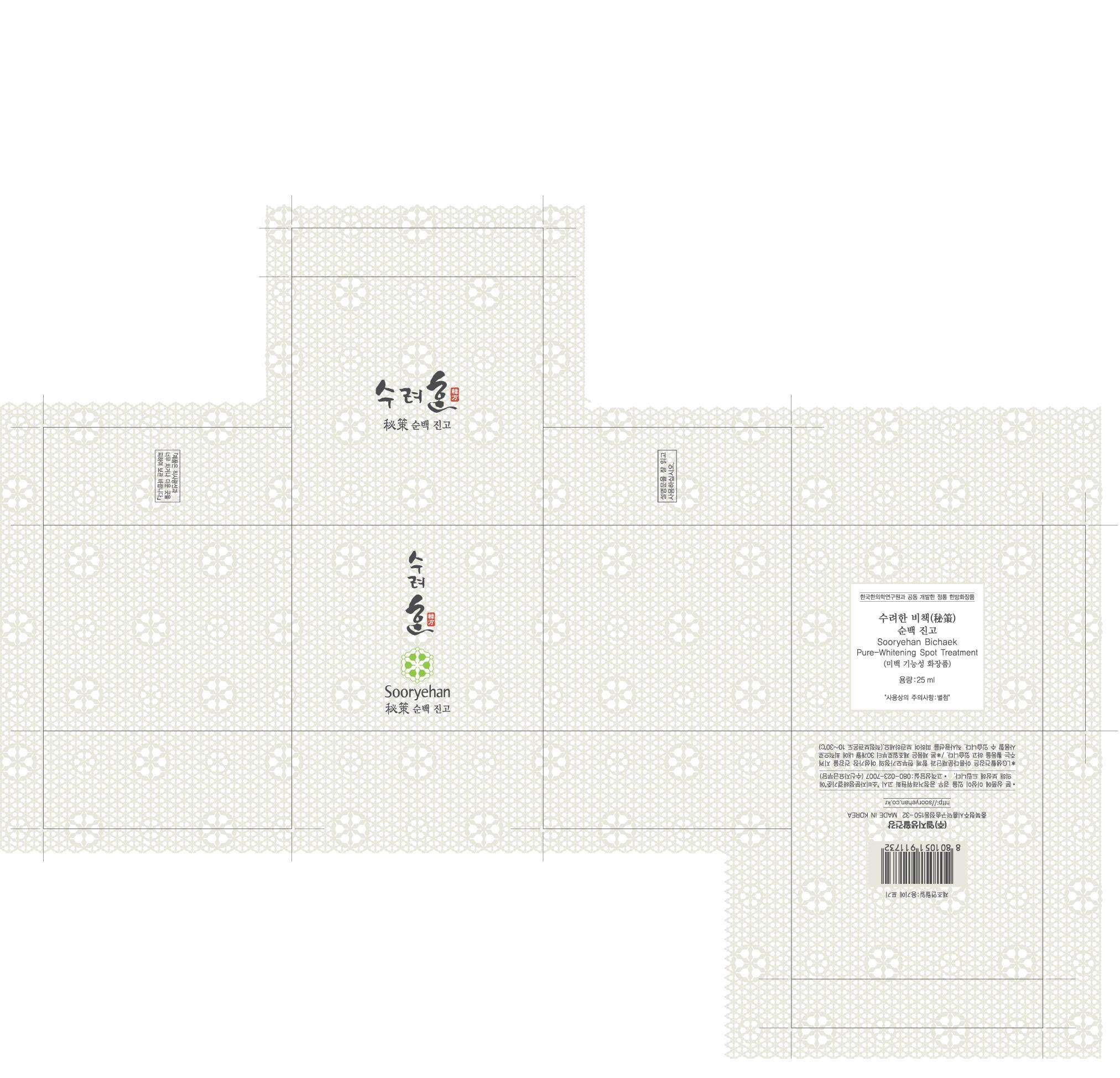
Sooryehan Pure Whitening Spot TreatmentPOLYDATIN CREAM
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!