Solutions
FULL PRESCRIBING INFORMATION
Active ingredient
Active Ingredient
OCTINOXATE 7.5%.........................................................
OCTISALATE 5.0%.........................................................
OXYBENZONE 4.0%.........................................................
AVOBENZONE 2.0%.......................................................
Purpose
Purpose
...............Sunscreen
...............Sunscreen
.............Sunscreen
..........Sunscreen
Uses
Uses
• helps prevent sunburn
Warnings
Skin Cancer/Skin Aging Alert: Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to help prevent sunburn, not skin cancer or early skin aging.
For external use only
Do not use on damaged or broken skin
When using this product keep out of eyes. Rinse with water to remove.
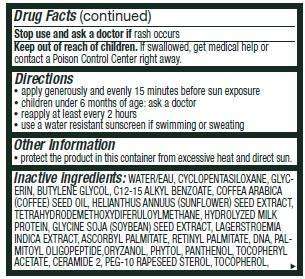
Stop use and ask a doctor if rash occurs
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
• apply liberally 15 minutes before sun exposure
• children under 6 months of age: ask a doctor
• reapply at least every 2 hours
• use a water resistant sunscreen if swimming or sweating
Other information
• protect the product in this container from excessive heat and direct sun
Inactive ingredients:
WATER/EAU, CYCLOPENTASILOXANE, GLYCERIN, BUTYLENE GLYCOL, C12-15 ALKYL BENZOATE, COFFEA ARABICA
(COFFEE) SEED OIL, HELIANTHUS ANNUUS (SUNFLOWER) SEED EXTRACT, TETRAHYDRODEMETHOXYDIFERULOYLMETHANE, HYDROLYZED MILK
PROTEIN, GLYCINE SOJA (SOYBEAN) SEED EXTRACT, LAGERSTROEMIA INDICA EXTRACT, ASCORBYL PALMITATE, RETINYL PALMITATE, DNA, PALMITOYL OLIGOPEPTIDE,ORYZANOL, PHYTOL, PANTHENOL, TOCOPHERYL ACETATE, CERAMIDE 2, PEG-10 RAPESEED STEROL, TOCOPHEROL, DIMETHICONOL, DIMETHICONE, TRIMETHYLSILOXYSILICATE, TRIBEHENIN, PEG-100 STEARATE, CAPRYLIC/CAPRIC TRIGLYCERIDE PEG-4 ESTERS, LAURETH-4, SODIUM HYDROXIDE,DISODIUM EDTA, SILICA, CARBOMER, PHOSPHORIC ACID, TETRAHYDROBISDEMETHOXYDIFERULOYLMETHANE, HEXYLENE GLYCOL, PHENOXYETHANOL, CAPRYLYL GLYCOL, PARFUM/FRAGRANCE.
Questions or Comments?
Call toll free 1-800-FOR-AVON



SolutionsOCTINOXATE, OCTISALATE, OXYBENZONE, AVOBENZONE CREAM
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||