Home – Solidago Compositum
Solidago Compositum
Heel Inc
Solidago Compositum 2.2ml Oral Vial
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
ACTIVE INGREDIENTS
Active ingredients:PURPOSE
KEEP OUT OF REACH OF CHILDREN
Keep this and all medicines out of the reach of children.SOLIDAGO COMPOSITUM INDICATIONS AND USAGE
Indications:
- Minor urinary discomfort
- Pain and burning upon urination
- Urinary urgency.
WARNINGS
Warnings: SOLIDAGO COMPOSITUM DOSAGE AND ADMINISTRATION
Dosasge:INACTIVE INGREDIENTS
Inactive ingredients: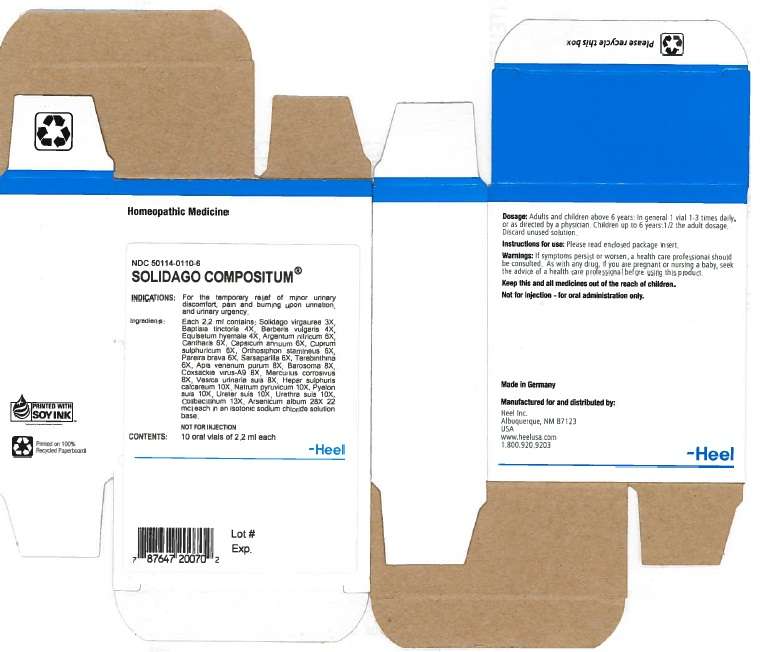
Solidago Compositum
SOLIDAGO VIRGAUREA FLOWERING TOP and BAPTISIA TINCTORIA ROOT and BERBERIS VULGARIS ROOT BARK and EQUISETUM HYEMALE and SILVER NITRATE and LYTTA VESICATORIA and CAPSICUM and CUPRIC SULFATE and CLERODENDRANTHUS SPICATUS LEAF and CHONDRODENDRON TOMENTOSUM ROOT and SMILAX REGELII ROOT and TURPENTINE OIL and APIS MELLIFERA VENOM and AGATHOSMA BETULINA LEAF and HUMAN COXSACKIEVIRUS A and MERCURIC CHLORIDE and SUS SCROFA URINARY BLADDER and CALCIUM SULFIDE and SODIUM PYRUVATE SOLUTION
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:50114-0110 |
|
Route of Administration
|
ORAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
2.2 in 1 VIAL |
|
|
|
2 |
NDC:50114-0110-6 |
10 in 1 CARTON |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
|
|
1993-01-31 |
|
|
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!
