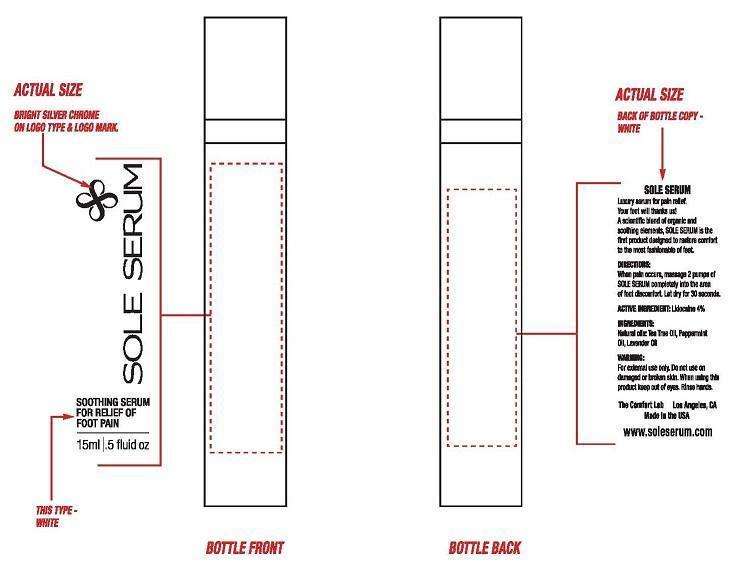
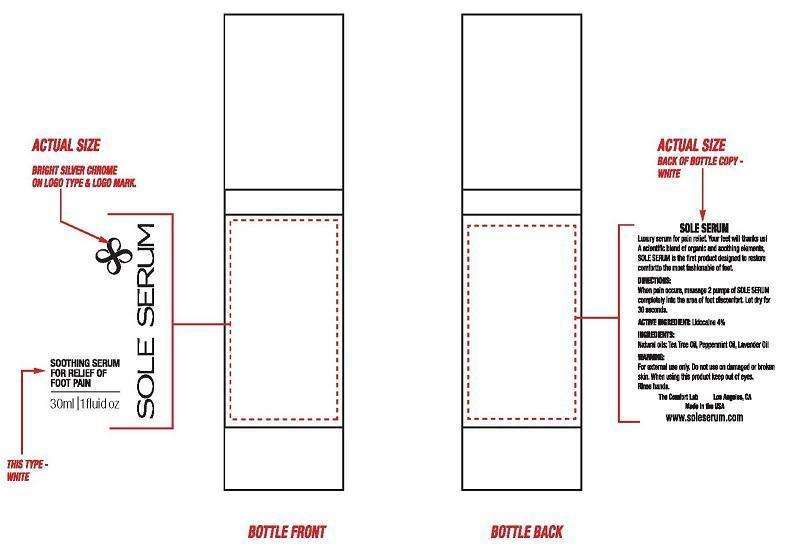
SOLE SERUM
THE COMFORT LAB LLC
THE COMFORT LAB LLC
FULL PRESCRIBING INFORMATION
Active ingredient
Active Ingredient
Lidocaine Hydrochloride 4%
Purpose
Purpose
External Analgesic
Uses
Use
For temporary relief of minor aches and pains of
muscles and joints.
Warnings
For external use only. If condition worsens, or if symptoms persist for more than 7 days or clear up and occur again within a few days, discontinue use of this product and consult a physician. Do not use in large quantities, particularly over raw surfaces or blistered areas. Avoid contact with the eyes. Rinse with water to remove.
KEEP OUT OF REACH OF CHILDREN.
Directions
Apply 2 pumps to foot where pain is felt. Apply to affected area not more than 3 to 4 times daily. Children under 2 years of age: consult a physician.
Inactive Ingredients
Acrylates/C10-30 Alkyl Acrylates Crosspolymer, Acrylates/Dimethicone Copolymer, Alkyl Benzoate, Diazolidinyl Urea, Ethoxydiglycol, Ethylenediaminetetraacetic acid, Disodium EDTA, Ethylhexyl Palmitate, Glyceryl Stearate, Peg 100 Stearate, Isododecane, Behenyl
Methacrylate/t-Butyl Methacrylate Copolymer, Isopropyl Palmitate, Lavandula Angustifolia (Lavender) Oil, Lecithin, Malaleuca Alternifolia {Tea
Tree) Leaf Oil, Mentha Piperita (Peppermint) Oil, Menthol, Peg-8 Dimethicone, Phenoxyethanol,Benzoic Acid, Ethylhexylglycerin, Glycereth -2 Cocoate, Poloxamer 407, Potassium Cetyl Phosphate, Potassium Sorbate, Propylene Glycol, Sorbic Acid, Water
Other Information
Provides penetrating foot pain relief


SOLE SERUMLIDOCAINE SPRAY
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||