SohMed Acid Reducer
SohMed™ Acid Reducer
FULL PRESCRIBING INFORMATION: CONTENTS*
- Use
- Warnings
- Directions
- SohMed Acid Reducer Other information
- Inactive ingredients
- Question or comments ?
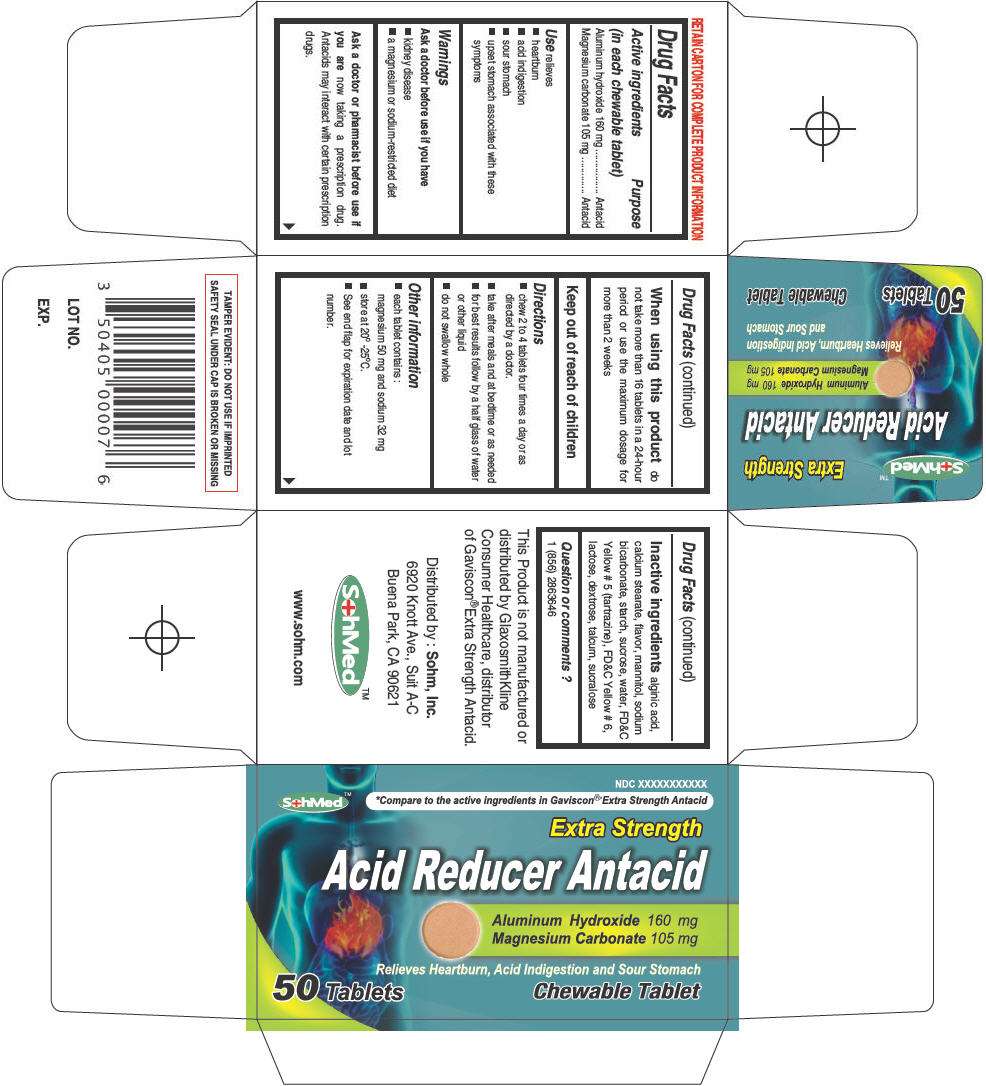
- PRINCIPAL DISPLAY PANEL - 50 Tablet Bottle Carton
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient
Purpose
|
Active ingredients (in each chewable tablet) |
Purpose |
|---|---|
| Aluminum hydroxide 160 mg | Antacid |
| Magnesium carbonate 105 mg | Antacid |
Use
relieves
- heartburn
- acid indigestion
- sour stomach
- upset stomach associated with these symptoms
Warnings
Ask a doctor before use if you have
- kidney disease
- a magnesium or sodium-restricted diet
Ask a doctor or pharmacist before use if you are now taking a prescription drug. Antacids may interact with certain prescription drugs.
When using this product do not take more than 16 tablets in a 24-hour period or use the maximum dosage for more than 2 weeks
Keep out of reach of children
Directions
- chew 2 to 4 tablets four times a day or as directed by a doctor.
- take after meals and at bedtime or as needed
- for best results follow by a half glass of water or other liquid
- do not swallow whole
SohMed Acid Reducer Other information
- each tablet contains :
magnesium 50 mg and sodium 32 mg - store at 20° -25°C.
- See end flap for expiration date and lot number.
Inactive ingredients
alginic acid, calcium stearate, flavor, mannitol, sodium bicarbonate, starch, sucrose, water, FD&C Yellow # 5 (tartrazine), FD&C Yellow # 6, lactose, dextrose, talcum, sucralose
Question or comments ?
1 (856) 2863646
Distributed by : Sohm, Inc.
6920 Knott Ave., Suit A-C
Buena Park, CA 90621
PRINCIPAL DISPLAY PANEL - 50 Tablet Bottle Carton
NDC XXXXXXXXXXX
SohMed™
*Compare to the active ingredients in Gaviscon® Extra Strength Antacid
Extra Strength
Acid Reducer Antacid
Aluminum Hydroxide 160 mg
Magnesium Carbonate 105 mg
Relieves Heartburn, Acid Indigestion and Sour Stomach
50 Tablets
Chewable Tablet
SohMed Acid ReducerALUMINUM HYDROXIDE and MAGNESIUM CARBONATE TABLET, CHEWABLE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||