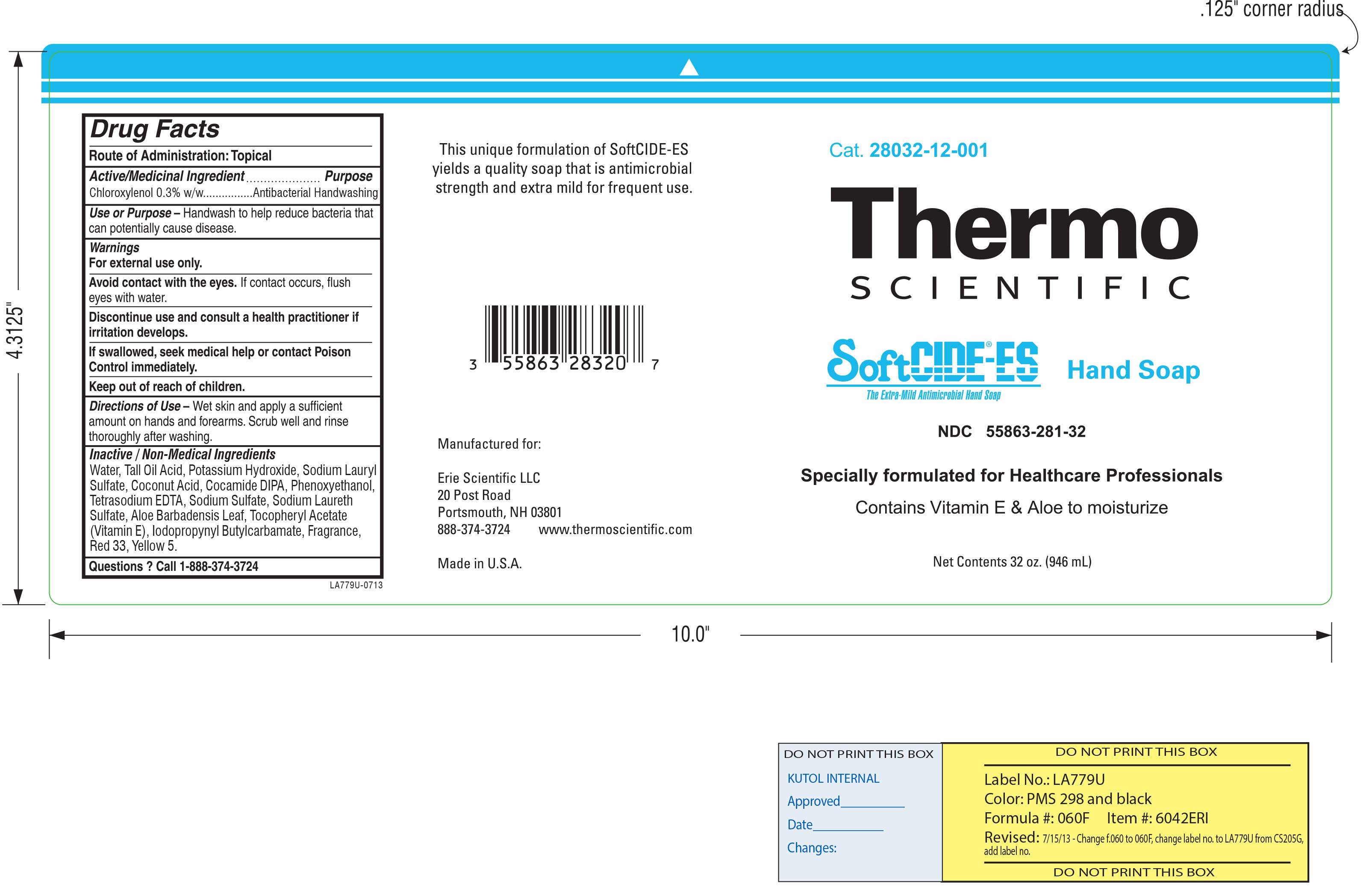
SoftCIDE-ES
Erie Scientific, LLC
Kutol Products Company, Inc.
SoftCIDE-ES
FULL PRESCRIBING INFORMATION
Active ingredient
Chloroxylenol 0.3% w/w
Water, Tall Oil Acid, Potassium Hydroxide, Sodium Lauryl Sulfate, Coconut Acid, Cocamide DIPA, Phenoxyethanol, Tetrasodium EDTA, Sodium Sulfate, Sodium Laureth Sulfate, Aloe Barbadensis Leaf, Tocopheryl Acetate (Vitamin E Acetate), Iodopropynyl Butylcarbamate, Fragrance, Red 33, Yellow 5
Wet skin and apply a sufficient amount on hands and forearms. Scrub well and rinse thoroughly after washing.
Purpose
Handwash to help reduce bacteria that can potentially cause disease.
Uses
Handwash to help reduce bacteria that can potentially cause disease.
Avoid contact with the eyes. If contact occurs, flush eyes with water.
Discontinue use and consult a health practitioner if irritation develops. If swallowed seek medical help or contact Posion Control immediately. Keep out of reach of children.
Keep out of reach of children. If swallowed, seek medical help or contact Poison Control immediately.
Avoid contact with eyes. If contact occurs, flush eyes with water.
For external use only.
Avoid contact with the eyes. If contact occurs, flush eyes with water.
Discontinue use and consult a health practitioner if irritation develops.
If swallowed seek medical help or contact Posion Control immediately.
Keep out of reach of children.
 55863-281-27-ERI.jpg
55863-281-27-ERI.jpg
 55863-281-32-ERI.jpg
55863-281-32-ERI.jpg
 55863-281-32-VWR.jpg
55863-281-32-VWR.jpg
 55863-281-27-VWR.jpg
55863-281-27-VWR.jpg
SoftCIDE-ESSoftCIDE-ES LIQUID
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||