Soft N Sure Foamed Antiseptic Handrub
Soft 'N Sure Foamed Antiseptic Handrub
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- Use
- Warnings
- Directions
- Inactive ingredients
- Questions or comments?
- PRINCIPAL DISPLAY PANEL - 482g Can Case
FULL PRESCRIBING INFORMATION
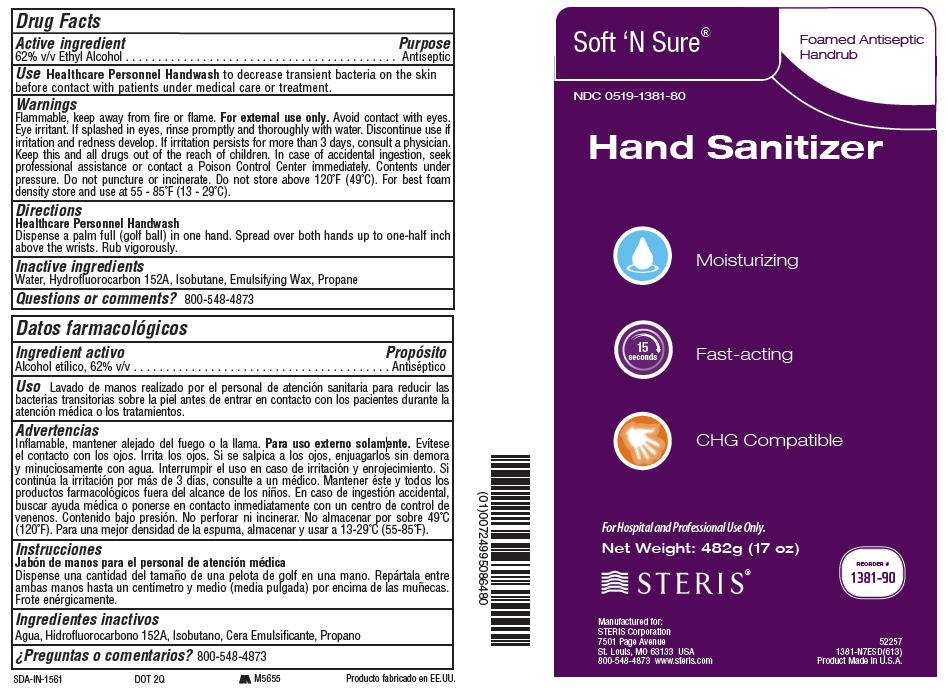
Drug Facts
Active ingredient
62% v/v Ethyl Alcohol
Purpose
Antiseptic
Use
Healthcare Personnel Handwash to decrease transient bacteria on the skin before contact with patients under medical care or treatment.
Warnings
Flammable, keep away from fire or flame. For external use only.
Avoid contact with eyes. Eye irritant. If splashed in eyes, rinse promptly and thoroughly with water.
Discontinue use if irritation and redness develop.
If irritation persists for more than 3 days, consult a physician.
Keep this and all drugs out of the reach of children. In case of accidental ingestion, seek professional assistance or contact a Poison Control Center immediately.
Contents under pressure. Do not puncture or incinerate. Do not store above 120°F (49°C). For best foam density store and use at 55 - 85°F (13 - 29°C).
Directions
Healthcare Personnel Handwash
Dispense a palm full (golf ball) in one hand. Spread over both hands up to one-half inch above the wrists. Rub vigorously.
Inactive ingredients
Water, Hydrofluorocarbon 152A, Isobutane, Emulsifying Wax, Propane
Questions or comments?
800-548-4873
PRINCIPAL DISPLAY PANEL - 482g Can Case
Soft 'N Sure®
Foamed Antiseptic
Handrub
NDC 0519-1381-80
Hand Sanitizer
Moisturizing
15
seconds
Fast-acting
CHG Compatible
For Hospital and Professional Use Only.
Net Weight: 482g (17 oz)
STERIS®
REORDER #
1381-90
Manufactured for:
STERIS Corporation
7501 Page Avenue
St. Louis, MO 63133 USA
800-548-4873 www.steris.com
52257
1381-N7ESD(613)
Product Made in U.S.A.
Soft N Sure Foamed Antiseptic HandrubALCOHOL AEROSOL, FOAM
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||