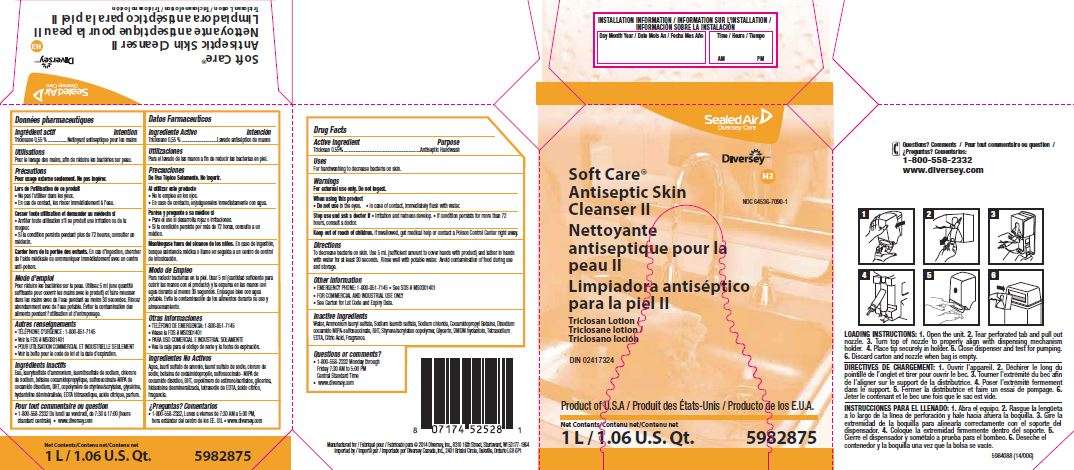
Soft Care Antiseptic Skin Cleanser II
Drug Facts
FULL PRESCRIBING INFORMATION
Active ingredient
Active ingredient
Triclosan 0.55%
Purpose
Purpose
Antiseptic handwash
Uses
Uses
For hand washing to decrease bacteria on skin.
Warnings
For external use only. Do not ingest.
When using this product
Do not use in the eyes.
In case of contact, immediately flush with water.
Stop use and ask a doctor if
irritation and redness develops.
If condition persists for more than 72 hours, consult a doctor.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
To decrease bacteria on skin.
Use 5 mL (sufficient amount to cover hands with product) and lather in hands with water for at least 30 seconds.
Rinse well with potable water.
Avoid contamination of food during use and storage.
Inactive ingredients
Water, Ammonium lauryl sulfate, Sodium laureth sulfate, Sodium chloride, Cocamidopropyl betaine, Disodium cocamido MIPA-sulfosuccinate, BHT, Styrene/acrylates copolymer, Glycerin, DMDM hydantoin, Tetrasodium EDTA, Citric acid, Fragrance
Questions or comments?
1-800-558-2332 Monday through Friday 7:30 AM to 5:00 PM
Central Standard Time
www.diversey.com
Sealed Air
Diversey Care
Diversey
H3
Soft Care
Antiseptic Skin
Cleanser II
NDC 64536-7090-1
Triclosan lotion
DIN 02417324
Product of U.S.A.
Net Contents
1 L/1.06 U.S. Qt.
5982875


Other information
EMERGENCY PHONE: 1-800-851-7145
See SDS # MS0301401
FOR COMMERCIAL AND INDUSTRIAL USE ONLY
See Carton for Lot Code and Expiry Date.
Soft Care Antiseptic Skin Cleanser IITRICLOSAN LIQUID
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||