Sodium Sulfacetamide
Sodium Sulfacetamide Medicated Pads 10% in a Urea Vehicle
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Topical Antibacterial Therapy
FOR DERMATOLOGICAL USE ONLY. NOT FOR OPHTHALMIC USE.
DESCRIPTION: 10% Sodium Sulfacetamide Medicated Pads contain 10% sodium sulfacetamide. Inactive ingredients: purified water, sodium thiosulfate, trisodium EDTA, and urea.
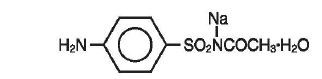
Sodium sulfacetamide is a sulfonamide with antibacterial activity. Chemically, sodium sulfacetamide is C8H9N2Na03S•H20, with a molecular weight of 254.24. Chemically it is Acetamide, N- [(4 aminophenyl) sulfonyl]-, monosodium salt and monohydrate, with the following structural formula:
CLINICAL PHARMACOLOGY: Sodium sulfacetamide exerts a bacteriostatic effect against sulfonamide-sensitive gram-positive and gram-negative microorganisms including Propionibacterium acnes commonly isolated from secondary cutaneous pyogenic infections.
The most widely accepted mechanism of action of sulfonamides is the Woods-Fildes theory, which is based on the fact that sulfonamides act as competitive antagonists to para-aminobenzoic acid (PABA), an essential component for bacterial growth. While absorption through intact skin in humans has not been determined, sodium sulfacetamide is readily absorbed from the gastrointestinal tract when taken orally and excreted in the urine largely unchanged. The biological half-life has variously been reported as 7 to 12.8 hours.
Uses
INDICATIONS AND USAGE: 10% Sodium Sulfacetamide Medicated Pads are indicated for the treatment of bacterial infections of the skin due to organisms susceptible to sulfonamides including Propionibacterium acnes. They are also intended for topical control of seborrheic dermatitis of the face and scalp.
CONTRAINDICATIONS: 10% Sodium Sulfacetamide Medicated Pads are contraindicated in persons with known or suspected hypersensitivity to sulfonamides or to any of the ingredients of the preparation. 10% Sodium Sulfacetamide Medicated Pads are not to be used by patients with kidney disease.
WARNINGS: Although rare, sensitivity to sodium sulfacetamide may occur. Therefore, caution and careful supervision should be observed when prescribing this drug for patients who may be prone to hypersensitivity to topical sulfonamides. Systemic toxic reactions such as agranulocytosis, acute hemolytic anemia, purpura hemorrhagica, drug fever, jaundice, and contact dermatitis indicate hypersensitivity to sulfonamides. Sulfonamides are known to cause Stevens-Johnson syndrome in hypersensitive individuals. Stevens-Johnson syndrome also has been reported following the topical use of sodium sulfacetamide. Cases of drug-induced systemic lupus erythematosus from topical sulfacetamide have also been reported.
PRECAUTIONS: General: Nonsusceptible organisms, including fungi, may proliferate with the use of this preparation. Hypersensitivity reactions may recur when a sulfonamide is readministered, irrespective of the route of administration, and cross hypersensitivity between different sulfonamides may occur. If 10% Sodium Sulfacetamide Medicated Pads produce signs of hypersensitivity or other untoward reactions, discontinue use of the preparation. Systemic absorption of topical sulfonamides is greater following application to large, infected, abraded, denuded, or severely burned areas. Under these circumstances, any of the adverse effects produced by the systemic administration of these agents could potentially occur, and appropriate observations and laboratory determinations should be performed.
Information For Patients: Avoid contact with eyes, eyelids, lips, and mucous membranes. If accidental contact occurs, rinse with water. Patients should discontinue the use of 10% Sodium Sulfacetamide Medicated Pads if the condition becomes worse, or if a rash develops in the area being treated or elsewhere. 10% Sodium Sulfacetamide Medicated Pads also should be discontinued promptly and the physician notified if any arthritis, fever, or sores in the mouth develop.
Drug Interactions: 10% Sodium Sulfacetamide Medicated Pads are incompatible with silver preparations.
Carcinogenesis, Mutagenesis, Impairment of Fertility: Long-term animal studies for carcinogenic potential have not been performed on 10% Sodium Sulfacetamide Medicated Pads.
Pregnancy Category C: Animal reproduction studies have not been conducted with 10% Sodium Sulfacetamide Medicated Pads. It also is not known whether 10% Sodium Sulfacetamide Medicated Pads can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. 10% Sodium Sulfacetamide Medicated Pads should be used by a pregnant woman only if clearly needed.
Nursing Mothers: It is not known whether this drug is excreted in human milk following topical use of 10% Sodium Sulfacetamide Medicated Pads. Because many drugs are excreted in human milk, caution should be exercised when 10% Sodium Sulfacetamide Medicated Pads are administered to a nursing woman.
Pediatric Use: Safety and effectiveness in children below the age of 12 years have not been established.
ADVERSE REACTIONS: Reports of irritation and hypersensitivity to sodium sulfacetamide are uncommon. The following adverse reactions, reported after administration of sterile ophthalmic sodium sulfacetamide, are noteworthy: instances of Stevens-Johnson syndrome and instances of local hypersensitivity, which progressed to a syndrome resembling systemic lupus erythematosus; in one case a fatal outcome has been reported (see WARNINGS .)
Call your doctor for medical advice about side effects.
DOSAGE AND ADMINISTRATION: Cutaneous Bacterial Infection - Gently apply 10% Sodium Sulfacetamide Medicated Pads to the affected areas 1 to 2 times daily or as directed by a physician until the infection has cleared.
Seborrheic dermatitis - Gently apply 10% Sodium Sulfacetamide Medicated Pads to the skin or scalp 1 to 2 times daily or as directed by a physician.
Dispense in accordance with applicable state law. This product has not been approved by the FDA, is not listed in the Orange Book, and has not been approved or rated for therapeutic equivalence with any other product.
HOW SUPPLIED: 10% Sodium Sulfacetamide Medicated Pads, NDC 42808-0101-30.
Store at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F). See USP Controlled Room Temperature. Protect from freezing.
Note: 10% Sodium Sulfacetamide Medicated Pads may darken after prolonged storage. Slight discoloration does not impair the efficacy or safety of the product.
Rx Only
Manufactured in the U.S.A. for Exact-Rx, Inc., Melville, NY 11747
00-0101-30-205-00
Iss:5/11
PACKAGE LABEL -30 foil pouches
For External Use Only
NDC 42808-0101-30 Rx Only
Sodium Sulfacetamide
(In a Urea Vehicle)
10%
PADS
Exact-Rx.
INCORPORATED
Contains (30) foil pouches
each with a single-use 2.5 mL medicated pad

Sodium SulfacetamideSODIUM SULFACETAMIDE SWAB
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||