SODIUM IODIDE I 123
Sodium Iodide I-123 Capsules
FULL PRESCRIBING INFORMATION: CONTENTS*
- SODIUM IODIDE I 123 DESCRIPTION
- Physical Characteristics
- External Radiation
- CLINICAL PHARMACOLOGY
- SODIUM IODIDE I 123 INDICATIONS AND USAGE
- SODIUM IODIDE I 123 CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- SODIUM IODIDE I 123 ADVERSE REACTIONS
- SODIUM IODIDE I 123 DOSAGE AND ADMINISTRATION
- Radiation Dosimetry
- HOW SUPPLIED
- Storage and Handling
- PRINCIPAL DISPLAY PANEL - A601C0
- PRINCIPAL DISPLAY PANEL - A602C0
FULL PRESCRIBING INFORMATION
Rx Only.
Diagnostic-For Oral Administration
SODIUM IODIDE I 123 DESCRIPTION
Sodium Iodide I-123 (Na123I) for diagnostic use is supplied in capsules for oral administration. The capsules are available in strengths of 3.7 and 7.4 megabecquerels (MBq) (100 and 200 μCi) I-123 at time of calibration.
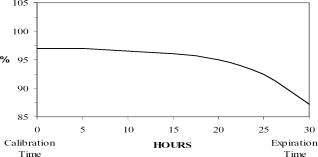
The radionuclidic composition at calibration is not less than 97.0 percent I-123, not more than 2.9 percent I-125 and not more than 0.1 percent Te-121. The radionuclidic composition at expiration time is not less than 87.2 percent I-123, not more than 12.4 percent I-125 and not more than 0.4 percent Te-121. The ratio of the concentration of I-123 and I-125 changes with time. Graph 1 shows the minimum concentration of I-123 as a function of time and Graph 2 shows the maximum concentration of I-125 as a function of time.
Graph 1. Radionuclidic Concentration of I-123
PERCENT OF TOTAL RADIOACTIVITY
IODINE-123
Physical Characteristics
Iodine-123 decays by electron capture with a physical half-life of 13.2 hours
| Radiation | Mean % Disintegration |
Energy (keV) |
| Gamma-2 | 83.4 | 159 |
External Radiation
The specific gamma ray constant for I-123 is 1.6 R/hr-mCi at 1 cm. The first half-value thickness of lead (Pb) for I-123 is 0.005 cm. A range of values for the relative attenuation of the radiation emitted by this radionuclide that results from the interposition of various thicknesses of Pb is shown in Table 2. For example, the use of 1.63 cm of lead will decrease the external radiation exposure by a factor of about 1,000.
| Shield Thickness (Pb), cm |
Coefficient of Attenuation |
| 0.005 0.10 0.88 1.63 2.48 |
0.5 10-1 10-2 10-3 10-4 |
Note that these estimates of attenuation do not take into consideration the presence of radionuclidic contaminants.
To correct for physical decay of I-123, the fractions that remain at selected intervals after the time of calibration are shown in Table 3.
| Hours |
Fraction Remaining |
Hours |
Fraction Remaining | |
|
*Time of Calibration |
||||
| 0* 3 6 9 12 15 |
1.000 0.854 0.730 0.623 0.533 0.455 |
18 21 24 27 30 |
0.389 0.332 0.284 0.242 0.207 |
|
CLINICAL PHARMACOLOGY
Sodium Iodide I-123 is readily absorbed from the upper gastrointestinal tract. Following absorption, the iodide is distributed primarily within the extracellular fluid of the body. It is trapped and organically bound by the thyroid and concentrated by the stomach, choroid plexus and salivary glands. It is excreted by the kidneys.
The fraction of the administered dose which is accumulated in the thyroid gland may be a measure of thyroid function in the absence of unusually high or low iodine intake or administration of certain drugs which influence iodine accumulation by the thyroid gland. Accordingly, the patient should be questioned carefully regarding previous medications and/or procedures involving radiographic media. Normal subjects can accumulate approximately 10 to 50% of the administered iodine dose in the thyroid gland, however, the normal and abnormal ranges are established by individual physician's criteria. The mapping (imaging) of Sodium Iodide I-123 distribution in the thyroid gland may provide useful information concerning thyroid anatomy and definition of normal and/or abnormal functioning of tissue within the gland.
SODIUM IODIDE I 123 INDICATIONS AND USAGE
Administration of Sodium Iodide I-123 is indicated as a diagnostic procedure to be used in evaluating thyroid function and/or morphology.
SODIUM IODIDE I 123 CONTRAINDICATIONS
To date there are no known contraindications to the use of Sodium Iodide I-123 capsules.
WARNINGS
Females of childbearing age and pediatric patients should not be studied unless the benefits anticipated from the performance of the test outweigh the possible risk of exposure to the amount of ionizing radiation associated with the test.
PRECAUTIONS
General
The contents of the capsule are radioactive. Adequate shielding of the preparation must be maintained at all times.
Do not use after the expiration time and date stated on the label.
The prescribed Sodium Iodide I-123 dose should be administered as soon as practical from the time of receipt of product (i.e., as close to calibration time as possible), in order to minimize the fraction of radiation exposure due to the relative increase of radionuclidic contaminants with time.
Sodium Iodide I-123, as well as other radioactive drugs, must be handled with care and appropriate safety measures should be used to minimize radiation exposure to clinical personnel. Care should also be taken to minimize radiation exposure to the patient consistent with proper patient management.
Radiopharmaceuticals should be used only by physicians who are qualified by training and experience in the safe use and handling of radionuclides, and whose experience and training have been approved by the appropriate government agency authorized to license the use of radionuclides.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term animal studies have been performed to evaluate carcinogenic potential, mutagenic potential, or whether Sodium Iodide I-123 affects fertility in males or females.
Pregnancy Category C
Animal reproduction studies have not been conducted with this drug. It is also not known whether Sodium Iodide I-123 can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. Sodium Iodide I-123 should be given to a pregnant woman only if clearly needed.
Ideally, examinations using radiopharmaceuticals, especially those elective in nature, in women of child-bearing capability should be performed during the first few (approximately ten) days following the onset of menses.
Nursing Mothers
Since I-123 is excreted in human milk, formula-feeding should be substituted for breast-feeding if the agent must be administered to the mother during lactation.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
SODIUM IODIDE I 123 ADVERSE REACTIONS
Although rare, reactions associated with the administration of Sodium Iodide isotopes for diagnostic use include, in decreasing order of frequency, nausea, vomiting, chest pain, tachycardia, itching skin, rash and hives.
SODIUM IODIDE I 123 DOSAGE AND ADMINISTRATION
The recommended oral dose for the average patient (70 kg) is 3.7 to 14.8 MBq (100 to 400 μCi). The lower part of the dosage range 3.7 MBq (100 μCi) is recommended for uptake studies alone, and the higher part 14.8 MBq (400 μCi) for thyroid imaging. The determination of I-123 concentration in the thyroid gland may be initiated at six hours after administering the dose and should be measured in accordance with standardized procedures.
The patient dose should be measured by a suitable radioactivity calibration system immediately prior to administration. The capsules can be utilized up to 30 hours after calibration time and date. Thereafter, discard the capsules in accordance with standard safety procedures. The user should wear waterproof gloves at all times when handling the capsules or container.
Radiation Dosimetry
The estimated absorbed radiation doses to several organs of an average patient (70 kg) from oral administration of the maximum dose of 14.8 MBq (400 μCi) of I-123 are shown in Table 4 for thyroid uptakes of 5, 15, and 25%. For comparison at these three values of thyroid uptake, the estimated radiation doses from doses of 3.7 MBq (100 μCi) I-131, also used as thyroid imaging agent, are also included.
| Estimated Radiation Absorbed Doses | ||||
| I-123 mGy/14.8 MBq (rads/400 μCi) |
I-131 mGy/3.7 MBq (rads/100 μCi) |
|||
| Target Organ | Maximum Thyroid Uptake (%) |
TOC | TOE | |
|
*Concentration at Time of Calibration: 97% I-123, 2.9% I-125, 0.1% Te-121 |
||||
|
Concentration at Time of Expiry: 87.2% I-123, 12.4% I-125, 0.4% Te-121 |
||||
|
Metabolic model in MIRD Dose Estimate Report 5 followed for I-123 and I-125 |
||||
|
Metabolic model in ICRP 30 followed for Te-121 |
||||
|
†Bladder voiding interval 4.8 hours. |
||||
| Bladder† | 5 | 1.7 (0.17) | 1.7 (0.17) | 2.9 (0.29) |
| 15 | 1.6 (0.16) | 1.6 (0.16) | 2.7 (0.27) | |
| 25 | 1.4 (0.14) | 1.5 (0.15) | 2.4 (0.24) | |
| Stomach Wall | 5 | 0.96 (0.096) | 0.98 (0.098) | 1.7 (0.17) |
| 15 | 0.89 (0.089) | 0.91 (0.091) | 1.5 (0.15) | |
| 25 | 0.82 (0.082) | 0.85 (0.085) | 1.4 (0.14) | |
| Small Intestine | 5 | 0.70 (0.070) | 0.71 (0.071) | 1.2 (0.12) |
| 15 | 0.65 (0.065) | 0.67 (0.067) | 1.1 (0.11) | |
| 25 | 0.60 (0.060) | 0.62 (0.062) | 0.99 (0.099) | |
| Liver | 5 | 0.089 (0.0089) | 0.13 (0.013) | 0.16 (0.016) |
| 15 | 0.10 (0.010) | 0.18 (0.018) | 0.28 (0.028) | |
| 25 | 0.11 (0.011) | 0.24 (0.024) | 0.41 (0.041) | |
| Ovaries | 5 | 0.18 (0.018) | 0.19 (0.019) | 0.18 (0.018) |
| 15 | 0.17 (0.017) | 0.18 (0.018) | 0.18 (0.018) | |
| 25 | 0.16 (0.016) | 0.18 (0.018) | 0.17 (0.017) | |
| Skeleton | 5 | 0.11 (0.011) | 0.16 (0.016) | 0.12 (0.012) |
| 15 | 0.12 (0.012) | 0.18 (0.018) | 0.18 (0.018) | |
| 25 | 0.14 (0.014) | 0.21 (0.021) | 0.24 (0.024) | |
| Red Marrow | 5 | 0.12 (0.012) | 0.16 (0.016) | 0.15 (0.015) |
| 15 | 0.12 (0.012) | 0.18 (0.018) | 0.21 (0.021) | |
| 25 | 0.13 (0.013) | 0.19 (0.019) | 0.27 (0.027) | |
| Testes | 5 | 0.076 (0.0076) | 0.089 (0.0089) | 0.12 (0.012) |
| 15 | 0.072 (0.0072) | 0.087 (0.0087) | 0.12 (0.012) | |
| 25 | 0.068 (0.0068) | 0.085 (0.0085) | 0.12 (0.012) | |
| Thyroid | 5 | 25 (2.5) | 75 (7.5) | 260 (26) |
| 15 | 77 (7.7) | 230 (23) | 780 (78) | |
| 25 | 130 (13) | 410 (41) | 1300 (130) | |
| Total Body | 5 | 0.11 (0.011) | 0.16 (0.016) | 0.24 (0.024) |
| 15 | 0.14 (0.014) | 0.25 (0.025) | 0.47 (0.047) | |
| 25 | 0.17 (0.017) | 0.35 (0.035) | 0.70 (0.070) | |
HOW SUPPLIED
Catalog Number 601,602.
Sodium Iodide I-123 is supplied as capsules for oral administration in strengths of 3.7 MBq (100 μCi) (red and white) (NDC 0019-N601-10) and 7.4 MBq (200 μCi) (green and white) (NDC 0019-N602-20) at time of calibration. Each gelatin capsule contains sucrose as a filler. The capsules are packaged in plastic vials containing one capsule of a single strength per vial. The plastic vial is packaged in a lead shield. A package insert is supplied with each lead shield.
Storage and Handling
The contents of the vial are radioactive and adequate shielding and handling precautions must be maintained.
Dispense and preserve capsules in tightly-closed containers that are adequately shielded. Store at controlled room temperature 20° to 25°C (68° to 77°F).
Storage and disposal of Sodium Iodide I-123 Capsules should be controlled in a manner that is in compliance with the appropriate regulations of the government agency authorized to license the use of this radionuclide.
Rev 101206
Mallinckrodt Inc.
St. Louis, MO 63134
tyco
Healthcare
MALLINCKRODT
A601I0
PRINCIPAL DISPLAY PANEL - A601C0
SODIUM IODIDE I 123 CAPSULES
DIAGNOSTIC
For Oral Administration Only
Store at Controlled Room Temperature
20-25°C (68-77°F)
READ PACKAGE INSERT FOR DIRECTIONS FOR USE
Rx Only.
WARNING: Radioactive drugs must be handled only by qualified personnel in conformity with regulations of the U.S. Nuclear Regulatory Commission or state regulatory agencies where applicable. Bottle containing drug should be kept in this container or within a heavier shield.
In Canada:
Mallinckrodt Canada Inc.
Pointe-Claire, Qc
Est. Lic. No. 100107-A
Mallinckrodt Inc.
St. Louis, MO 63134 USA
MALLINCKRODT
CAUTION RADIOACTIVE MATERIAL
A601C0
R9/2000
PRINCIPAL DISPLAY PANEL - A602C0
SODIUM IODIDE I 123 CAPSULES
DIAGNOSTIC
For Oral Administration Only
Store at Controlled Room Temperature
20-25°C (68-77°F)
READ PACKAGE INSERT FOR DIRECTIONS FOR USE
Rx Only.
WARNING: Radioactive drugs must be handled only by qualified personnel in conformity with regulations of the U.S. Nuclear Regulatory Commission or state regulatory agencies where applicable. Bottle containing drug should be kept in this container or within a heavier shield.
In Canada:
Mallinckrodt Canada Inc.
Pointe-Claire, Qc
Est. Lic. No. 100107-A
Mallinckrodt Inc.
St. Louis, MO 63134 USA
MALLINCKRODT
CAUTION RADIOACTIVE MATERIAL
A602C0
R9/2000

SODIUM IODIDE I 123sodium iodide i 123 CAPSULE, GELATIN COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
SODIUM IODIDE I 123sodium iodide i 123 CAPSULE, GELATIN COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||