SNAYTOX HYDROGEL LIP
NATURE REPUBLIC CO., LTD.
NATURE REPUBLIC CO., LTD.
FULL PRESCRIBING INFORMATION: CONTENTS*
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- SNAYTOX HYDROGEL LIP INDICATIONS AND USAGE
- SNAYTOX HYDROGEL LIP DOSAGE AND ADMINISTRATION
- Package Label. Principal Display Panel
FULL PRESCRIBING INFORMATION
ACTIVE INGREDIENT
Active Ingredients:
Hamamelis Virginiana (Witch Hazel) Extract (1%) - Skin Protectant
INACTIVE INGREDIENT
Inactive Ingredients:
Water, Glycerin, Chondrus Crispus (Carrageenan), Butylene Glycol, Scutellaria Baicalensis Root Extract, Paeonia Suffruticosa Root Extract, Ceratonia Siliqua Gum, Agar, Dipotassium Glycyrrhizate, Benzophenone-4, Lac Color, Hydrolyzed Collagen, Flavor,
Dipeptide Diaminobutyroyl Benzylamide Diacetate, Lycium Barbarum Fruit Extract, Centella Asiatica Extract, Daucus Carota Sativa (Carrot) Root Extract
PURPOSE
Purpose: Mildly moisturizing lip patch.
WARNINGS
Cautions:
For external use only.
Avoid contact with eyes and mouth.
Discontinue use if signs of irritation or rashes appear.
Keep out of reach of children.
Replace the cap after use.
KEEP OUT OF REACH OF CHILDREN
KEEP OUT OF REACH OF CHILDREN
SNAYTOX HYDROGEL LIP INDICATIONS AND USAGE
How to Use:
1. Remove the patch from the tray, and attach to the entire lips.
2. Leave the patch for 10-20 minutes.
3. Remove the patch, and gently pat to absorb the residue.
- Use the patch twice a week to maximize the results.
SNAYTOX HYDROGEL LIP DOSAGE AND ADMINISTRATION
How to Use:
1. Remove the patch from the tray, and attach to the entire lips.
2. Leave the patch for 10-20 minutes.
3. Remove the patch, and gently pat to absorb the residue.
- Use the patch twice a week to maximize the results.
Package Label. Principal Display Panel
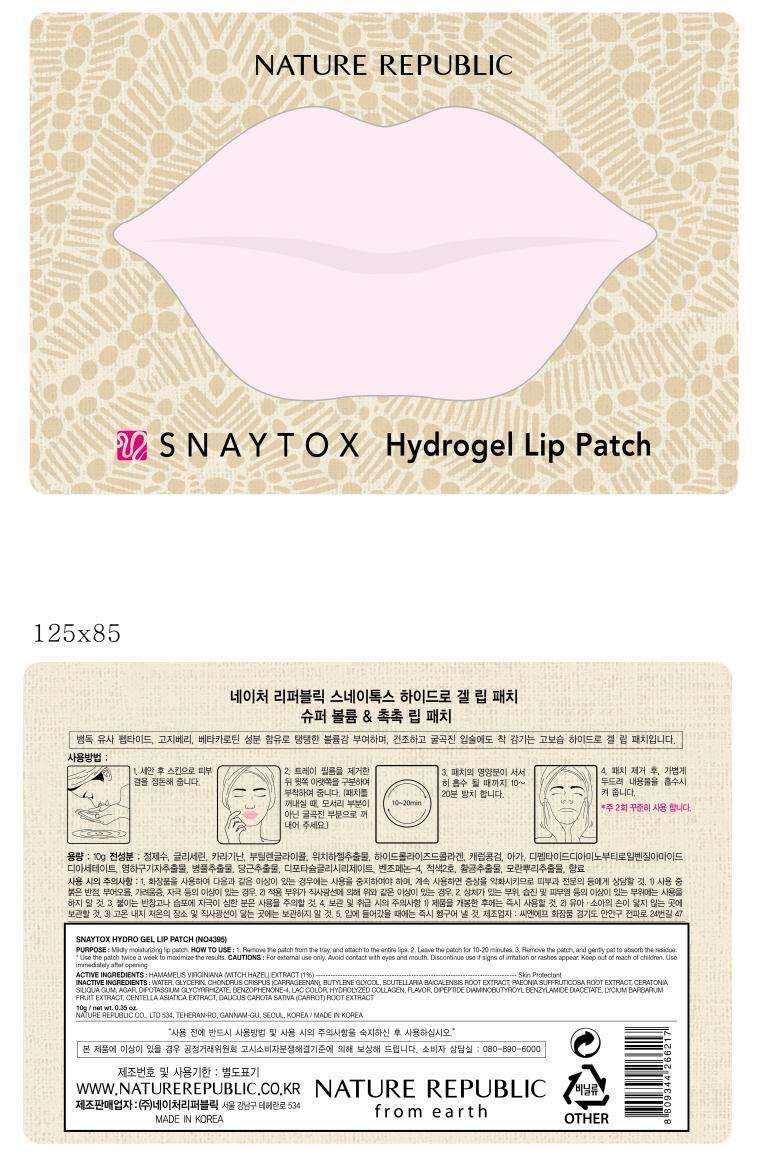
SNAYTOX HYDROGEL LIPWitch Hazel PATCH
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||