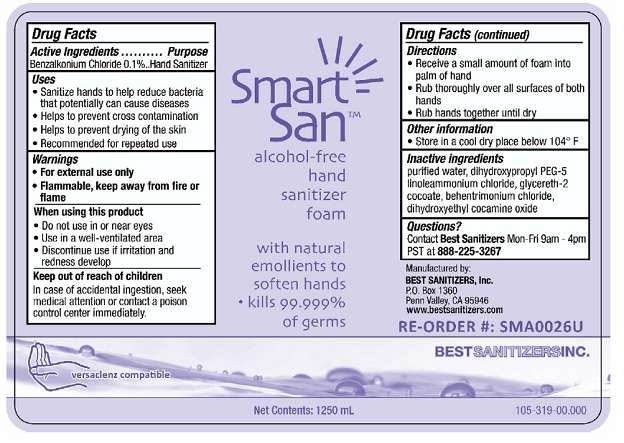
Smart San Alcohol Free Hand Sanitizer Foam
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredient
- Purpose
- Smart San Alcohol Free Hand Sanitizer Foam Uses
- Warnings
- When Using this product
- Keep out of reach of children
- Directions
- Smart San Alcohol Free Hand Sanitizer Foam Other information
- Inactive ingredients
- Questions?
- Principal Display Panel
FULL PRESCRIBING INFORMATION
Active Ingredient
Benzalkonium Chloride 0.1%
Purpose
Hand Sanitizer
Smart San Alcohol Free Hand Sanitizer Foam Uses
- Sanitize Hands to help reduce bacteria that potentially can causes disease
- Helps to prevent cross contamination
- Helps to prevent drying of the skin
- Recommended for repeated use
Warnings
- For external use only
-
Flammable, keep away from fire or flame
When Using this product
- Do not use in or near eyes
- Use in a well-ventilated area
- Discontinue use if irritation and redness develop
Keep out of reach of children
In case of accidental ingestion, seek medical attention or contact a poison control center immediately.
Directions
- Receive a small amount of foam into palm of hand
- Rub thoroughly over all surfaces of both hands
- Rub hands together until dry
Smart San Alcohol Free Hand Sanitizer Foam Other information
Store in a cool dry place below 104° F
Inactive ingredients
purified water, dihydroxypropyl PEG-5, linoleammonium chloride, glycereth-2 cocoate, behentrimonium chloride, dihydroxyethyl cocamine oxide
Questions?
Contact Best Sanitizers Mon-Fri 9am-4pm PST at 888-225-3267
Principal Display Panel
NDC 59900-118-06
NDC 59900-118-12
NDC 59900-118-48
Smart San™ alcohol-free hand sanitizer foam
Smart San Alcohol Free Hand Sanitizer FoamBenzalkonium Chloride LIQUID
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!