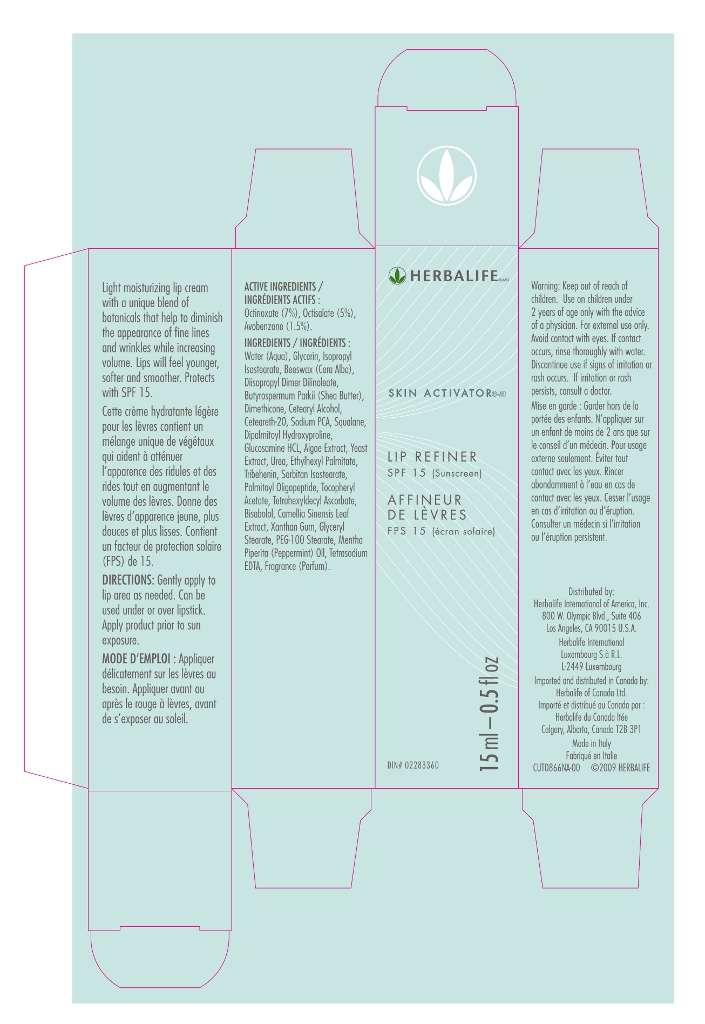
Skin Activator
Drug Facts
FULL PRESCRIBING INFORMATION
Active ingredient
ACTIVE INGREDIENTS
Octinoxate (7%)
Octisalate (5%)
Avobenzone (1.5%)
DIRECTIONS
Smooth onto lips daily.
Apply prior to sun exposure
WARNINGS
For external use only.
Keep out of reach of children.
Use on children under 2 years of age only with the advice of physician.
Avoid contact with eyes. If contact occurs, rinse thoroughly with water.
Discontinue use if signs of irritation or rush occurs. If irritation or rash persist, consult a doctor.
INACTIVE INGREDIENTS
Water
Glycerin
Isopropyl isostearate
Beeswax (cera alba)
Diisopropyl dimer dilinoleate
Butyrospermum parkii (shea) butter
Dimethicone
Cetearyl alcohol
Ceteareth 20
Sodium pca
Squalane
Dipalmitoyl hydroxyproline
Glucosamine hydrochloride
Algae extract
Yeast extract
Urea
Ethylhexyl palmitate
Tribehenin
Sorbitan isostearate
Palmitoyl oligopeptide
Tocopherol acetate
Tetrahexyldecyl ascorbate
Bisabolol
Camellia sinensis leaf extract
Xanthan gum
Glyceryl stearate
Peg 100 stearate
Mentha piperita (peppermint) oil
Tetrasodium edta
Fragrance (parfum)

Skin ActivatorOctinoxate, Octisalate and Avobenzone CREAM
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||