Sinsin Mulpas
SINSIN PHARMACEUTICAL CO., LTD.
SINSIN PHARMACEUTICAL CO., LTD.
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredients
- Purpose
- Sinsin Mulpas Uses
- Warnings
- For external use only.
- Do not use
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children.
- Directions
- Inactive ingredients
- Manufactured by:
- Principal Display Panel
FULL PRESCRIBING INFORMATION
Active ingredients
DL-Camphor 4.5%
L-Menthol 4.2%
Methyl Salicylate 6.9%
Purpose
Topical Analgesic
Sinsin Mulpas Uses
for temporary relief of minor aches and pains of muscles and joints associated with:
- arthritis
- simple backache
- strains
- bruises
- sprains
Warnings
For external use only.
Flammable
keep away from fire, sparks and heated surfaces
Do not use
- on wounds or damaged skin
When using this product
- do not bandage tightly
- avoid contact with the eyes mucous membranes
Stop use and ask a doctor if
condition worsens or symptoms persist for more than 7 days or clear up and occur again within a few days
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Adults and children 12 years of age and over:- adults and children 2 years of age and older: apply to the affected area no more than 3 to 4 times daily.
- children under 2 years of age: consult a doctor
- Consult a doctor.
Inactive ingredients
Concentrated Glycerin, Chlorpheniramine Maleate, Ethanol (synthetic), Purified Water, Thymol
Manufactured by:
SINSIN PHARM. CO., LTD.
90, Beomjigi-ro 141 beon-gil, Danwon-Gu, Ansan-si, Gyeonggi-do, Korea
http://www.sinsin.com tel: 82-31-776-1140~3
Principal Display Panel
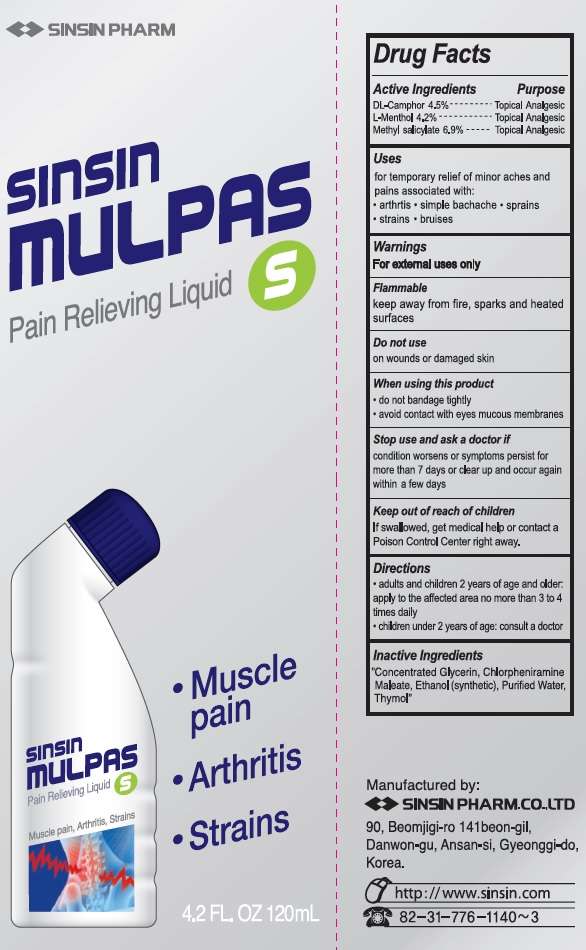
Sinsin MulpasCamphor, Menthol, Methyl Salicylate LIQUID
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||