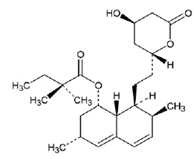
Simvastatin
NCS HealthCare of KY, Inc dba Vangard Labs
HIGHLIGHTS OF PRESCRIBING INFORMATION RECENT MAJOR CHANGESDosage and Administration Recommended Dosing (2.1) 06/2011 Restricted Dosing for 80 mg (2.2) 06/2011 Coadministration with Other Drugs (2.3) 10/2011 Patients with Homozygous Familial Hypercholesterolemia (2.4) 06/2011 Chinese Patients Taking Lipid-Modifying Doses (≥1 g/day Niacin) of Niacin-Containing Products (2.7) 06/2011Contraindications (4) 02/2012Warnings and Precautions Myopathy/Rhabdomyolysis (5.1) 02/2012 Liver Dysfunction (5.2) 10/2011 Endocrine Function (5.3) 10/2011INDICATIONS AND USAGESimvastatin tablets, USP are an HMG-CoA reductase inhibitor (statin) indicated as an adjunctive therapy to diet to: Reduce the risk of total mortality by reducing CHD deaths and reduce the risk of non-fatal myocardial infarction, stroke, and the need for revascularization procedures in patients at high risk of coronary events. (1.1) Reduce elevated total-C, LDL-C, Apo B, TG and increase HDL-C in patients with primary hyperlipidemia (heterozygous familial and nonfamilial) and mixed dyslipidemia. (1.2) Reduce elevated TG in patients with hypertriglyceridemia and reduce TG and VLDL-C in patients with primary dysbetalipoproteinemia. (1.2) Reduce total-C and LDL-C in adult patients with homozygous familial hypercholesterolemia. (1.2) Reduce elevated total-C, LDL-C, and Apo B in boys and postmenarchal girls, 10 to 17 years of age with heterozygous familial hypercholesterolemia after failing an adequate trial of diet therapy. (1.2, 1.3) Limitations of Use Simvastatin tablets, USP have not been studied in Fredrickson Types I and V dyslipidemias. (1.4)DOSAGE AND ADMINISTRATION Dose range is 5 to 40 mg/day. (2.1) Recommended usual starting dose is 10 or 20 mg once a day in the evening. (2.1) Recommended starting dose for patients at high risk of CHD is 40 mg/day. (2.1) Due to the increased risk of myopathy, including rhabdomyolysis, use of the 80 mg dose of simvastatin tablets should be restricted to patients who have been taking simvastatin 80 mg chronically (e.g., for 12 months or more) without evidence of muscle toxicity. (2.2) Patients who are currently tolerating the 80 mg dose of simvastatin tablets who need to be initiated on an interacting drug that is contraindicated or is associated with a dose cap for simvastatin should be switched to an alternative statin with less potential for the drug-drug interaction. (2.2) Due to the increased risk of myopathy, including rhabdomyolysis, associated with the 80 mg dose of simvastatin tablets, patients unable to achieve their LDL-C goal utilizing the 40 mg dose of simvastatin tablets should not be titrated to the 80 mg dose, but should be placed on alternative LDL-C-lowering treatment(s) that provides greater LDL-C lowering. (2.2) Adolescents (10 to 17 years of age) with HeFH: Starting dose is 10 mg/day; maximum recommended dose is 40 mg/day. (2.5) DOSAGE FORMS AND STRENGTHSCONTRAINDICATIONS Concomitant administration of strong CYP3A4 inhibitors. (4, 5.1) Concomitant administration of gemfibrozil, cyclosporine, or danazol. (4, 5.1) Hypersensitivity to any component of this medication. (4, 6.2) Active liver disease, which may include unexplained persistent elevations in hepatic transaminase levels. (4, 5.2) Women who are pregnant or may become pregnant. (4, 8.1) Nursing mothers. (4, 8.3) WARNINGS AND PRECAUTIONS Patients should be advised of the increased risk of myopathy including rhabdomyolysis with the 80 mg dose. (5.1) Skeletal muscle effects (e.g., myopathy and rhabdomyolysis): Risks increase with higher doses and concomitant use of certain medicines. Predisposing factors include advanced age (≥65), female gender, uncontrolled hypothyroidism, and renal impairment. (4, 5.1, 8.5, 8.6) Patients should be advised to report promptly any symptoms of myopathy. Simvastatin therapy should be discontinued immediately if myopathy is diagnosed or suspected. See Drug Interaction table. (5.1) Liver enzyme abnormalities: Persistent elevations in hepatic transaminases can occur. Check liver enzyme tests before initiating therapy and as clinically indicated thereafter. (5.2) Side EffectsDRUG INTERACTIONS Drug Interactions Associated with Increased Risk of Myopathy/Rhabdomyolysis (2.3, 4, 5.1, 7.1, 7.2, 7.3, 12.3) Interacting Agents Prescribing Recommendations Strong CYP3A4 inhibitors (e.g.,Itraconazole, ketoconazole, posaconazole, erythromycin, clarithromycin, telithromycin, HIV protease inhibitors, boceprevir, telaprevir, nefazodone), gemfibrozil, cyclosporine, danazol Contraindicated with simvastatin Verapamil, diltiazem Do not exceed 10 mg simvastatin daily Amiodarone, amlodipine, ranolazine Do not exceed 20 mg simvastatin daily Grapefruit juice Avoid large quantities of grapefruit juice (>1 quart daily) Other Lipid-lowering Medications: Use with other fibrate products or lipid-modifying doses (≥1 g/day) of niacin increases the risk of adverse skeletal muscle effects. Caution should be used when prescribing with simvastatin. (5.1, 7.2, 7.4) Coumarin anticoagulants: Concomitant use with simvastatin prolongs INR. Achieve stable INR prior to starting simvastatin. Monitor INR frequently until stable upon initiation or alteration of simvastatin therapy. (7.6) USE IN SPECIFIC POPULATIONS
FULL PRESCRIBING INFORMATION: CONTENTS*
- 3 DOSAGE FORMS AND STRENGTHS
- 4 SIMVASTATIN CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- PRINCIPAL DISPLAY PANEL - 80MG
FULL PRESCRIBING INFORMATION
In patients at high risk of coronary events because of existing coronary heart disease, diabetes, peripheral vessel disease, history of stroke or other cerebrovascular disease, simvastatin tablets, USP are indicated to:
- Reduce the risk of total mortality by reducing CHD deaths.
- Reduce the risk of non-fatal myocardial infarction and stroke.
- Reduce the need for coronary and non-coronary revascularization procedures.
- Reduce elevated total cholesterol (total-C), low-density lipoprotein cholesterol (LDL-C), apolipoprotein B (Apo B), and triglycerides (TG), and to increase high-density lipoprotein cholesterol (HDL-C) in patients with primary hyperlipidemia (Fredrickson type IIa, heterozygous familial and nonfamilial) or mixed dyslipidemia (Fredrickson type IIb).
- Reduce elevated TG in patients with hypertriglyceridemia (Fredrickson type IV hyperlipidemia).
- Reduce elevated TG and VLDL-C in patients with primary dysbetalipoproteinemia (Fredrickson type III hyperlipidemia).
- Reduce total-C and LDL-C in patients with homozygous familial hypercholesterolemia as an adjunct to other lipid-lowering treatments (e.g., LDL apheresis) or if such treatments are unavailable.
- LDL cholesterol remains ≥190 mg/dL; or
- LDL cholesterol remains ≥160 mg/dL and
- There is a positive family history of premature cardiovascular disease (CVD) or
- Two or more other CVD risk factors are present in the adolescent patient.
see Warnings and Precautions
Patients taking Verapamil or Diltiazem
- The dose of simvastatin tablets should not exceed 10 mg/day [see Warnings and Precautions (5.1), Drug Interactions (7.3), and Clinical Pharmacology (12.3)].
Patients taking Amiodarone, Amlodipine or Ranolazine
- The dose of simvastatin tablets should not exceed 20 mg/day [see Warnings and Precautions (5.1), Drug Interactions (7.3), and Clinical Pharmacology (12.3)].
see Dosage and Administration, Restricted Dosing for 80 mg
Clinical Studies
see Warnings and Precautionsand Clinical Pharmacology
Because of an increased risk for myopathy in Chinese patients taking simvastatin 40 mg coadministered with lipid-modifying doses (≥1 g/day niacin) of niacin-containing products, caution should be used when treating Chinese patients with simvastatin doses exceeding 20 mg/day coadministered with lipid-modifying doses of niacin-containing products. Because the risk for myopathy is dose-related, Chinese patients should not receive simvastatin 80 mg coadministered with lipid-modifying doses of niacin-containing products. The cause of the increased risk of myopathy is not known. It is also unknown if the risk for myopathy with coadministration of simvastatin with lipid-modifying doses of niacin-containing products observed in Chinese patients applies to other Asian patients. [See Warnings and Precautions (5.1).]
3 DOSAGE FORMS AND STRENGTHS
- Tablets simvastatin 5 mg are yellow colored, round shaped, biconvex, film coated tablets, debossed with ‘A’ on one side and ‘15’ on the other side.
- Tablets simvastatin 10 mg are light pink colored, round shaped, biconvex, film coated tablets, debossed with ‘A’ on one side and ‘01’ on the other side.
- Tablets simvastatin 20 mg are light pink colored, round shaped, biconvex, film coated tablets, debossed with ‘A’ on one side and ‘02’ on the other side.
- Tablets simvastatin 40 mg are pink colored, round shaped, biconvex, film coated tablets, debossed with ‘A’ on one side and ‘03’ on the other side.
- Tablets simvastatin 80 mg are pink colored, capsule shaped, biconvex, film coated tablets, debossed with ‘A’ on one side and ‘04’ on the other side.
4 CONTRAINDICATIONS
Simvastatin tablets are contraindicated in the following conditions:
- Concomitant administration of strong CYP3A4 inhibitors (e.g., itraconazole, ketoconazole, posaconazole, HIV protease inhibitors, boceprevir, telaprevir, erythromycin, clarithromycin, telithromycin and nefazodone) [see Warnings and Precautions (5.1)].
- Concomitant administration of gemfibrozil, cyclosporine, or danazol [see Warnings and Precautions (5.1)].
- Hypersensitivity to any component of this medication [see Adverse Reactions (6.2)].
- Active liver disease, which may include unexplained persistent elevations in hepatic transaminase levels [see Warnings and Precautions (5.2)].
- Women who are pregnant or may become pregnant. Serum cholesterol and triglycerides increase during normal pregnancy, and cholesterol or cholesterol derivatives are essential for fetal development. Because HMG-CoA reductase inhibitors (statins) decrease cholesterol synthesis and possibly the synthesis of other biologically active substances derived from cholesterol, simvastatin tablets may cause fetal harm when administered to a pregnant woman. Atherosclerosis is a chronic process and the discontinuation of lipid-lowering drugs during pregnancy should have little impact on the outcome of long-term therapy of primary hypercholesterolemia. There are no adequate and well-controlled studies of use with simvastatin tablets during pregnancy; however, in rare reports congenital anomalies were observed following intrauterine exposure to statins. In rat and rabbit animal reproduction studies, simvastatin revealed no evidence of teratogenicity. Simvastatin tablets should be administered to women of childbearing age only when such patients are highly unlikely to conceive. If the patient becomes pregnant while taking this drug, simvastatin tablets should be discontinued immediately and the patient should be apprised of the potential hazard to the fetus [see Use in Specific Populations (8.1)].
- Nursing mothers. It is not known whether simvastatin is excreted into human milk; however, a small amount of another drug in this class does pass into breast milk. Because statins have the potential for serious adverse reactions in nursing infants, women who require treatment with simvastatin tablets should not breastfeed their infants [see Use in Specific Populations (8.3)].
5 WARNINGS AND PRECAUTIONS
5.1 Myopathy/Rhabdomyolysis
The risk of myopathy, including rhabdomyolysis, is dose related.
The risk of myopathy, including rhabdomyolysis, is greater in patients on simvastatin 80 mg compared with other statin therapies with similar or greater LDL-C-lowering efficacy and compared with lower doses of simvastatin. Therefore, the 80 mg dose of simvastatin should be used only in patients who have been taking simvastatin 80 mg chronically (e.g., for 12 months or more) without evidence of muscle toxicity [see Dosage and Administration, Restricted Dosing for 80 mg (2.2)]. If, however, a patient who is currently tolerating the 80 mg dose of simvastatin needs to be initiated on an interacting drug that is contraindicated or is associated with a dose cap for simvastatin, that patient should be switched to an alternative statin with less potential for the drug-drug interaction. Patients should be advised of the increased risk of myopathy, including rhabdomyolysis, and to report promptly any unexplained muscle pain, tenderness or weakness. If symptoms occur, treatment should be discontinued immediately.See Warnings and Precautions
All patients starting therapy with simvastatin, or whose dose of simvastatin is being increased, should be advised of the risk of myopathy, including rhabdomyolysis, and told to report promptly any unexplained muscle pain, tenderness or weakness. Simvastatin therapy should be discontinued immediately if myopathy is diagnosed or suspected.
See Contraindicationsand Drug InteractionsSee Drug Interactions
see Contraindicationsand Drug Interactions
see Drug Interactions
see Drug Interactions
see Drug Interactionsand Table 3 in Clinical Pharmacology
see Drug Interactions
see also Dosage and AdministrationDrug InteractionsClinical Pharmacology
|
Interacting Agents |
Prescribing Recommendations |
|
Strong CYP3A4 Inhibitors e.g.,: Itraconazole Ketoconazole Posaconazole Erythromycin Clarithromycin Telithromycin HIV protease inhibitors Boceprevir Telaprevir Nefazodone Gemfibrozil Cyclosporine Danazol |
Contraindicated with simvastatin |
|
Verapamil Diltiazem |
Do not exceed 10 mg simvastatin daily |
|
Amiodarone Amlodipine Ranolazine |
Do not exceed 20 mg simvastatin daily |
|
Grapefruit juice |
Avoid large quantities of grapefruit juice (>1 quart daily) |
Persistent increases (to more than 3X the ULN) in serum transaminases have occurred in approximately 1% of patients who received simvastatin in clinical studies.
It is recommended that liver function tests be performed before the initiation of treatment, and thereafter when clinically indicated.
Increases in HbA1c and fasting serum glucose levels have been reported with HMG-CoA reductase inhibitors, including simvastatin.
| Simvastatin (N = 2,221) % |
Placebo (N = 2,223) % |
|
| Body as a Whole Edema/swelling Abdominal pain |
2.7 5.9 |
2.3 5.8 |
| Cardiovascular System Disorders Atrial fibrillation |
5.7 |
5.1 |
| Digestive System Disorders Constipation Gastritis |
2.2 4.9 |
1.6 3.9 |
| Endocrine Disorders Diabetes mellitus |
4.2 |
3.6 |
| Musculoskeletal Disorders Myalgia |
3.7 |
3.2 |
| Nervous System/Psychiatric Disorders Headache Insomnia Vertigo |
2.5 4 4.5 |
2.1 3.8 4.2 |
| Respiratory System Disorders Bronchitis Sinusitis |
6.6 2.3 |
6.3 1.8 |
| Skin/Skin Appendage Disorders Eczema |
4.5 |
3 |
| Urogenital System Disorders Infection, urinary tract |
3.2 |
3.1 |
see Contraindicationsand Warnings and Precautions
see Warnings and Precautions
Doses greater than 40 mg have not been studied in this population.
Of the 2,423 patients who received simvastatin in Phase III clinical studies and the 10,269 patients in the Heart Protection Study who received simvastatin, 363 (15%) and 5,366 (52%), respectively were ≥65 years old. In HPS, 615 (6%) were ≥75 years old. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. Since advanced age (≥65 years) is a predisposing factor for myopathy, simvastatin should be prescribed with caution in the elderly. [See Clinical Pharmacology (12.3).]
A pharmacokinetic study with simvastatin showed the mean plasma level of statin activity to be approximately 45% higher in elderly patients between 70 to 78 years of age compared with patients between 18 to 30 years of age. In 4S, 1,021 (23%) of 4,444 patients were 65 or older. Lipid-lowering efficacy was at least as great in elderly patients compared with younger patients, and simvastatin significantly reduced total mortality and CHD mortality in elderly patients with a history of CHD. In HPS, 52% of patients were elderly (4,891 patients 65 to 69 years and 5,806 patients 70 years or older). The relative risk reductions of CHD death, non-fatal MI, coronary and non-coronary revascularization procedures, and stroke were similar in older and younger patients [see Clinical Studies (14.1)]. In HPS, among 32,145 patients entering the active run-in period, there were 2 cases of myopathy/rhabdomyolysis; these patients were aged 67 and 73. Of the 7 cases of myopathy/rhabdomyolysis among 10,269 patients allocated to simvastatin, 4 were aged 65 or more (at baseline), of whom one was over 75. There were no overall differences in safety between older and younger patients in either 4S or HPS.
Because advanced age (≥65 years) is a predisposing factor for myopathy, including rhabdomyolysis, simvastatin should be prescribed with caution in the elderly. In a clinical trial of patients treated with simvastatin 80 mg/day, patients ≥65 years of age had an increased risk of myopathy, including rhabdomyolysis, compared to patients <65 years of age. [See Warnings and Precautions (5.1) and Clinical Pharmacology (12.3).]
Simvastatin is contraindicated in patients with active liver disease which may include unexplained persistent elevations in hepatic transaminase levels [see Contraindications (4) and Warnings and Precautions (5.2)].
| * Results based on a chemical assay except results with propranolol as indicated. † Results could be representative of the following CYP3A4 inhibitors: ketoconazole, erythromycin, clarithromycin, HIV protease inhibitors, and nefazodone. ‡ Simvastatin acid refers to the β-hydroxyacid of simvastatin. § The effect of amounts of grapefruit juice between those used in these two studies on simvastatin pharmacokinetics has not been studied. ¶ Double-strength: one can of frozen concentrate diluted with one can of water. Grapefruit juice was administered TID for 2 days, and 200 mL together with single dose simvastatin and 30 and 90 minutes following single dose simvastatin on Day 3. # Single-strength: one can of frozen concentrate diluted with 3 cans of water. Grapefruit juice was administered with breakfast for 3 days, and simvastatin was administered in the evening on Day 3. Þ Because Chinese patients have an increased risk for myopathy with simvastatin coadministered with lipid-modifying doses (≥ 1 gram/day niacin) of niacin-containing products, and the risk is dose-related, Chinese patients should not receive simvastatin 80 mg coadministered with lipid-modifying doses of niacin-containing products [see Warnings and Precautions (5.1) and Drug Interactions (7.4)]. |
|||||
| Coadministered Drug or Grapefruit Juice |
Dosing of Coadministered Drug or Grapefruit Juice |
Dosing of Simvastatin |
Geometric Mean Ratio (Ratio* with/without coadministered drug) No Effect = 1 |
||
| AUC |
Cmax |
||||
| Contraindicated with simvastatin [see Contraindications (4) and Warnings and Precautions (5.1)] |
|||||
| Telithromycin† |
200 mg QD for 4 days |
80 mg |
simvastatin acid‡ simvastatin |
12 8.9 |
15 5.3 |
| Nelfinavir† |
1250 mg BID for 14 days |
20 mg QD for 28 days |
simvastatin acid‡ simvastatin |
6 |
6.2 |
| Itraconazole† |
200 mg QD for 4 days |
80 mg |
simvastatin acid‡ simvastatin |
13.1 13.1 |
|
| Posaconazole |
100 mg (oral suspension) QD for 13 days 200 mg (oral suspension) QD for 13 days |
40 mg 40 mg |
simvastatin acid simvastatin simvastatin acid simvastatin |
7.3 10.3 8.5 10.6 |
9.2 9.4 9.5 11.4 |
| Gemfibrozil |
600 mg BID for 3 days |
40 mg |
simvastatin acid simvastatin |
2.85 1.35 |
2.18 0.91 |
| Avoid >1 quart of grapefruit juice with simvastatin [see Warnings and Precautions (5.1)] |
|||||
| Grapefruit Juice§ (high dose) |
200 mL of double-strength TID¶ |
60 mg single dose |
simvastatin acid simvastatin |
7 16 |
|
| Grapefruit Juice§ (low dose) |
8 oz (about 237 mL) of single-strength# |
20 mg single dose |
simvastatin acid simvastatin |
1.3 1.9 |
|
| Avoid taking with >10 mg simvastatin, based on clinical and/or postmarketing experience [see Warnings and Precautions (5.1)] |
|||||
| Verapamil SR |
240 mg QD Days 1 to 7 then 240 mg BID on Days 8 to 10 |
80 mg on Day 10 |
simvastatin acid simvastatin |
2.3 2.5 |
2.4 2.1 |
| Diltiazem |
120 mg BID for 10 days |
80 mg on Day 10 |
simvastatin acid simvastatin |
2.69 3.1 |
2.69 2.88 |
| Diltiazem |
120 mg BID for 14 days |
20 mg on Day 14 |
simvastatin |
4.6 |
3.6 |
| Avoid taking with >20 mg simvastatin, based on clinical and/or postmarketing experience [see Warnings and Precautions (5.1)] | |||||
| Amiodarone |
400 mg QD for 3 days |
40 mg on Day 3 |
simvastatin acid simvastatin |
1.75 1.76 |
1.72 1.79 |
| Amlodipine |
10 mg QD x 10 days |
80 mg on Day 10 |
simvastatin acid simvastatin |
1.58 1.77 |
1.56 1.47 |
| Ranolazine SR |
1000 mg BID for 7 days |
80 mg on Day 1 and Day 6 to 9 |
simvastatin acid simvastatin |
2.26 1.86 |
2.28 1.75 |
| No dosing adjustments required for the following: |
|||||
| Fenofibrate |
160 mg QD x 14 days |
80 mg QD on Days 8 to 14 |
simvastatin acid simvastatin |
0.64 0.89 |
0.89 0.83 |
| Niacin extended-releaseÞ |
2 g single dose |
20 mg single dose |
simvastatin acid simvastatin |
1.6 1.4 |
1.84 1.08 |
| Propranolol |
80 mg single dose |
80 mg single dose |
total inhibitor active inhibitor |
0.79 0.79 |
↓ from 33.6 to 21.1 ng·eq/mL ↓ from 7 to 4.7 ng·eq/mL |
Reductions in Risk of CHD Mortality and Cardiovascular Events
In 4S, the effect of therapy with simvastatin on total mortality was assessed in 4,444 patients with CHD and baseline total cholesterol 212 to 309 mg/dL (5.5 to 8 mmol/L). In this multicenter, randomized, double-blind, placebo-controlled study, patients were treated with standard care, including diet, and either simvastatin 20 to 40 mg/day (n=2,221) or placebo (n=2,223) for a median duration of 5.4 years. Over the course of the study, treatment with simvastatin led to mean reductions in total-C, LDL-C and TG of 25%, 35%, and 10%, respectively, and a mean increase in HDL-C of 8%. Simvastatin significantly reduced the risk of mortality by 30% (p=0.0003, 182 deaths in the simvastatin group vs 256 deaths in the placebo group). The risk of CHD mortality was significantly reduced by 42% (p=0.00001, 111 vs 189 deaths). There was no statistically significant difference between groups in non-cardiovascular mortality. Simvastatin significantly decreased the risk of having major coronary events (CHD mortality plus hospital-verified and silent non-fatal myocardial infarction [MI]) by 34% (p<0.00001, 431 vs 622 patients with one or more events). The risk of having a hospital-verified non-fatal MI was reduced by 37%. Simvastatin significantly reduced the risk for undergoing myocardial revascularization procedures (coronary artery bypass grafting or percutaneous transluminal coronary angioplasty) by 37% (p<0.00001, 252 vs 383 patients). Simvastatin significantly reduced the risk of fatal plus non-fatal cerebrovascular events (combined stroke and transient ischemic attacks) by 28% (p=0.033, 75 vs 102 patients). Simvastatin reduced the risk of major coronary events to a similar extent across the range of baseline total and LDL cholesterol levels. Because there were only 53 female deaths, the effect of simvastatin on mortality in women could not be adequately assessed. However, simvastatin significantly lessened the risk of having major coronary events by 34% (60 vs 91 women with one or more event). The randomization was stratified by angina alone (21% of each treatment group) or a previous MI. Because there were only 57 deaths among the patients with angina alone at baseline, the effect of simvastatin on mortality in this subgroup could not be adequately assessed. However, trends in reduced coronary mortality, major coronary events and revascularization procedures were consistent between this group and the total study cohort. Additionally, simvastatin resulted in similar decreases in relative risk for total mortality, CHD mortality, and major coronary events in elderly patients (≥65 years), compared with younger patients.
The Heart Protection Study (HPS) was a large, multi-center, placebo-controlled, double-blind study with a mean duration of 5 years conducted in 20,536 patients (10,269 on simvastatin 40 mg and 10,267 on placebo). Patients were allocated to treatment using a covariate adaptive method3 which took into account the distribution of 10 important baseline characteristics of patients already enrolled and minimized the imbalance of those characteristics across the groups. Patients had a mean age of 64 years (range 40 to 80 years), were 97% Caucasian and were at high risk of developing a major coronary event because of existing CHD (65%), diabetes (Type 2, 26%; Type 1, 3%), history of stroke or other cerebrovascular disease (16%), peripheral vessel disease (33%), or hypertension in males ≥65 years (6%). At baseline, 3,421 patients (17%) had LDL-C levels below 100 mg/dL, of whom 953 (5%) had LDL-C levels below 80 mg/dL; 7,068 patients (34%) had levels between 100 and 130 mg/dL; and 10,047 patients (49%) had levels greater than 130 mg/dL.
The HPS results showed that simvastatin 40 mg/day significantly reduced: total and CHD mortality; non-fatal MI, stroke, and revascularization procedures (coronary and non-coronary) (see Table 4).
| † n = number of patients with indicated event |
||||
| Endpoint |
Simvastatin (N=10,269) n (%)† |
Placebo (N=10,267) n (%)† |
Risk Reduction (%) (95% CI) |
p-Value |
| Primary Mortality CHD mortality |
1,328 (12.9) 587 (5.7) |
1,507 (14.7) 707 (6.9) |
13 (6-19) 18 (8-26) |
p=0.0003 p=0.0005 |
| Secondary Non-fatal MI Stroke |
357 (3.5) 444 (4.3) |
574 (5.6) 585 (5.7) |
38 (30-46) 25 (15-34) |
p<0.0001 p<0.0001 |
| Tertiary Coronary revascularization Peripheral and other non-coronary revascularization |
513 (5) 450 (4.4) |
725 (7.1) 532 (5.2) |
30 (22-38) 16 (5-26) |
p<0.0001 p=0.006 |
Two composite endpoints were defined in order to have sufficient events to assess relative risk reductions across a range of baseline characteristics (see Figure 1). A composite of major coronary events (MCE) was comprised of CHD mortality and non-fatal MI (analyzed by time-to-first event; 898 patients treated with simvastatin had events and 1,212 patients on placebo had events). A composite of major vascular events (MVE) was comprised of MCE, stroke and revascularization procedures including coronary, peripheral and other non-coronary procedures (analyzed by time-to-first event; 2,033 patients treated with simvastatin had events and 2,585 patients on placebo had events). Significant relative risk reductions were observed for both composite endpoints (27% for MCE and 24% for MVE, p<0.0001). Treatment with simvastatin produced significant relative risk reductions for all components of the composite endpoints. The risk reductions produced by simvastatin in both MCE and MVE were evident and consistent regardless of cardiovascular disease related medical history at study entry (i.e., CHD alone; or peripheral vascular disease, cerebrovascular disease, diabetes or treated hypertension, with or without CHD), gender, age, creatinine levels up to the entry limit of 2.3 mg/dL, baseline levels of LDL-C, HDL-C, apolipoprotein B and A-1, baseline concomitant cardiovascular medications (i.e., aspirin, beta blockers, or calcium channel blockers), smoking status, alcohol intake, or obesity. Diabetics showed risk reductions for MCE and MVE due to simvastatin treatment regardless of baseline HbA1c levels or obesity with the greatest effects seen for diabetics without CHD.
Figure 1 The Effects of Treatment with Simvastatin on Major Vascular Events and Major Coronary Events in HPS
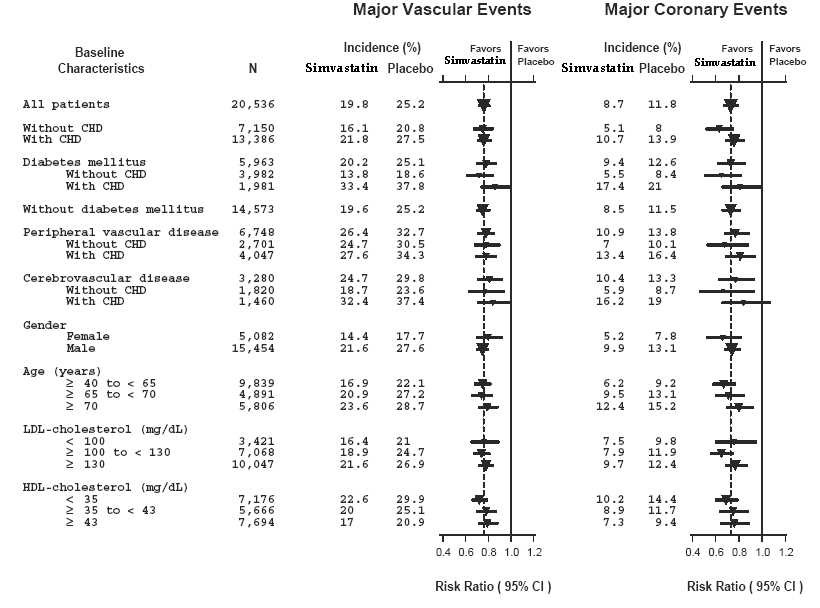
N = number of patients in each subgroup. The inverted triangles are point estimates of the relative risk, with their 95% confidence intervals represented as a line. The area of a triangle is proportional to the number of patients with MVE or MCE in the subgroup relative to the number with MVE or MCE, respectively, in the entire study population. The vertical solid line represents a relative risk of one. The vertical dashed line represents the point estimate of relative risk in the entire study population.
Angiographic Studies
In the Multicenter Anti-Atheroma Study, the effect of simvastatin on atherosclerosis was assessed by quantitative coronary angiography in hypercholesterolemic patients with CHD. In this randomized, double-blind, controlled study, patients were treated with simvastatin 20 mg/day or placebo. Angiograms were evaluated at baseline, two and four years. The co-primary study endpoints were mean change per-patient in minimum and mean lumen diameters, indicating focal and diffuse disease, respectively. Simvastatin significantly slowed the progression of lesions as measured in the Year 4 angiogram by both parameters, as well as by change in percent diameter stenosis. In addition, simvastatin significantly decreased the proportion of patients with new lesions and with new total occlusions.
Modifications of Lipid Profiles
Primary Hyperlipidemia (Fredrickson type lla and llb)
Simvastatin has been shown to be effective in reducing total-C and LDL-C in heterozygous familial and non-familial forms of hyperlipidemia and in mixed hyperlipidemia. Maximal to near maximal response is generally achieved within 4 to 6 weeks and maintained during chronic therapy. Simvastatin consistently and significantly decreased total-C, LDL-C, total-C/HDL-C ratio, and LDL-C/HDL-C ratio; simvastatin also decreased TG and increased HDL-C (see Table 5)
| † median percent change ‡ mean baseline LDL-C 244 mg/dL and median baseline TG 168 mg/dL § mean baseline LDL-C 188 mg/dL and median baseline TG 128 mg/dL || mean baseline LDL-C 226 mg/dL and median baseline TG 156 mg/dL ¶ 21% and 36% median reduction in TG in patients with TG ≤200 mg/dL and TG >200 mg/dL, respectively. Patients with TG >350 mg/dL were excluded †† mean baseline LDL-C 156 mg/dL and median baseline TG 391 mg/dL. |
|||||
| TREATMENT |
N |
TOTAL-C |
LDL-C |
HDL-C |
TG† |
| Lower Dose Comparative Study‡ (Mean % Change at Week 6) Simvastatin 5 mg q.p.m. Simvastatin 10 mg q.p.m. |
109 110 |
-19 -23 |
-26 -30 |
10 12 |
-12 -15 |
| Scandinavian Simvastatin Survival Study§ (Mean % Change at Week 6) Placebo Simvastatin 20 mg q.p.m. |
2223 2221 |
-1 -28 |
-1 -38 |
0 8 |
-2 -19 |
| Upper Dose Comparative Study|| (Mean % Change Averaged at Weeks 18 and 24) Simvastatin 40 mg q.p.m. Simvastatin 80 mg q.p.m.¶ |
433 664 |
-31 -36 |
-41 -47 |
9 8 |
-18 -24 |
| Multi-Center Combined Hyperlipidemia Study†† (Mean % Change at Week 6) Placebo Simvastatin 40 mg q.p.m Simvastatin 80 mg q.p.m |
125 123 124 |
1 -25 -31 |
2 -29 -36 |
3 13 16 |
-4 -28 -33 |
| † The median baseline values (mg/dL) for the patients in this study were: total-C = 254, LDL-C = 135, HDL-C = 36, TG = 404, VLDL-C = 83, and non-HDL-C = 215. |
|||||||
| TREATMENT |
N |
Total-C |
LDL-C |
HDL-C |
TG |
VLDL-C |
Non-HDL-C |
| Placebo |
74 |
+2 (-7, +7) |
+1 (-8, +14) |
+3 (-3, +10) |
-9 (-25, +13) |
-7 (-25, +11) |
+1 (-9, +8) |
| Simvastatin 40 mg/day |
74 |
-25 (-34, -19) |
-28 (-40, -17) |
+11 (+5, +23) |
-29 (-43, -16) |
-37 (-54, -23) |
-32 (-42, -23) |
| Simvastatin 80 mg/day |
74 |
-32 (-38, -24) |
-37 (-46, -26) |
+15 (+5, +23) |
-34 (-45, -18) |
-41 (-57, -28) |
-38 (-49, -32) |
| † The median baseline values (mg/dL) were: total-C = 324, LDL-C = 121, HDL-C = 31, TG = 411, VLDL-C = 170, and non-HDL-C = 291. |
|||||||
| TREATMENT |
N |
Total-C |
LDL-C + IDL |
HDL-C |
TG |
VLDL-C+IDL |
Non-HDL-C |
| Placebo |
7 |
-8 (-24, +34) |
-8 (-27, +23) |
-2 (-21, +16) |
+4 (-22, +90) |
-4 (-28, +78) |
-8 (-26, -39) |
| Simvastatin 40 mg/day |
7 |
-50 (-66, -39) |
-50 (-60, -31) |
+7 (-8, +23) |
-41 (-74, -16) |
-58 (-90, -37) |
-57 (-72, -44) |
| Simvastatin 80 mg/day |
7 |
-52 (-55, -41) |
-51 (-57, -28) |
+7 (-5, +29) |
-38 (-58, +2) |
-60 (-72, -39) |
-59 (-61, -46) |
| † median percent change |
||||||||
| Dosage |
Duration |
N |
Total-C |
LDL-C |
HDL-C |
TG† |
Apo B |
|
| Placebo |
24 Weeks |
67 |
% Change from Baseline (95% CI) |
1.6 (-2.2, 5.3) |
1.1 (-3.4, 5.5) |
3.6 (-0.7, 8) |
-3.2 (-11.8, 5.4) |
-0.5 (-4.7, 3.6) |
| Mean baseline, mg/dL (SD) |
278.6 (51.8) |
211.9 (49) |
46.9 (11.9) |
90 (50.7) |
186.3 (38.1) |
|||
| Simvastatin |
24 Weeks |
106 |
% Change from Baseline (95% CI) |
-26.5 (-29.6, -23.3) |
-36.8 (-40.5, -33) |
8.3 (4.6, 11.9) |
-7.9 (-15.8, 0) |
-32.4 (-35.9, -29) |
| Mean baseline, mg/dL (SD) |
270.2 (44) |
203.8 (41.5) |
47.7 (9) |
78.3 (46) |
179.9 (33.8) |
|||
Simvastatin Tablets USP, 5 mg are yellow colored, round shaped, biconvex, film coated tablets, debossed with ‘A’ on one side and ‘15’ on the other side. They are supplied as follows:
Blistercards of 30 NDC 0615-7704-39
Simvastatin Tablets USP, 10 mg are light pink colored, round shaped, biconvex, film coated tablets, debossed with ‘A’ on one side and ‘01’ on the other side. They are supplied as follows:
Blistercards of 30 NDC 0615--7705-39
Simvastatin Tablets USP, 20 mg are light pink colored, round shaped, biconvex, film coated tablets, debossed with ‘A’ on one side and ‘02’ on the other side. They are supplied as follows:
Blistercards of 30 NDC 0615-7706-39
Simvastatin Tablets USP, 40 mg are pink colored, round shaped, biconvex, film coated tablets, debossed with ‘A’ on one side and ‘03’ on the other side. They are supplied as follows:
Blistercards of 30 NDC 0615-7707-39
Simvastatin Tablets USP, 80 mg are pink colored, capsule shaped, biconvex, film coated tablets, debossed with ‘A’ on one side and ‘04’ on the other side. They are supplied as follows:
Blistercards of 30 NDC 0615-7708-39
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Patients should be advised about substances they should not take concomitantly with simvastatin [see Contraindications (4) and Warnings and Precautions (5.1)]. Patients should also be advised to inform other healthcare professionals prescribing a new medication or increasing the dose of an existing medication that they are taking simvastatin.
Patients using the 80 mg dose should be informed that the risk of myopathy, including rhabdomyolysis, is increased with use of the 80 mg dose.
Simvastatin Tablets, USP 5mg

Simvastatin Tablets, USP 10mg
Simvastatin Tablets, USP 40mg
PRINCIPAL DISPLAY PANEL - 80MG
SIMVASTATIN 80 MG

SimvastatinSimvastatin TABLET, FILM COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
SimvastatinSimvastatin TABLET, FILM COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
SimvastatinSimvastatin TABLET, FILM COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
SimvastatinSimvastatin TABLET, FILM COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
SimvastatinSimvastatin TABLET, FILM COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||