SHISEIDO SUNCARE ULTIMATE
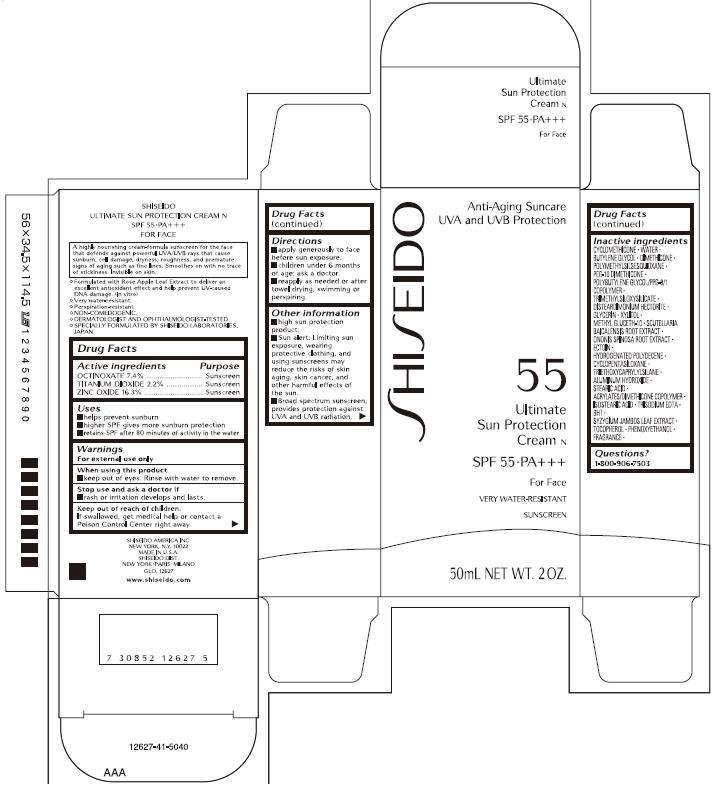
SHISEIDO ULTIMATE SUN PROTECTION CREAM N SPF 55•PA+++ FOR FACE
FULL PRESCRIBING INFORMATION: CONTENTS*
- SHISEIDO SUNCARE ULTIMATE Uses
- Warnings
- Directions
- SHISEIDO SUNCARE ULTIMATE Other information
- Inactive ingredients
- Questions?
- PRINCIPAL DISPLAY PANEL - 50mL Carton
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient
Purpose
| Active ingredients | Purpose |
| OCTINOXATE 7.4% | Sunscreen |
| TITANIUM DIOXIDE 2.2% | Sunscreen |
| ZINC OXIDE 16.3% | Sunscreen |
SHISEIDO SUNCARE ULTIMATE Uses
- helps prevent sunburn
- higher SPF gives more sunburn protection
- retains SPF after 80 minutes of activity in the water
Warnings
For external use only
When using this product
- keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if
- rash or irritation develops and lasts.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- apply generously to face before sun exposure.
- children under 6 months of age: ask a doctor.
- reapply as needed or after towel drying, swimming or perspiring.
SHISEIDO SUNCARE ULTIMATE Other information
- high sun protection product.
- Sun alert: Limiting sun exposure, wearing protective clothing, and using sunscreens may reduce the risks of skin aging, skin cancer, and other harmful effects of the sun.
- Broad spectrum sunscreen; provides protection against UVA and UVB radiation.
Inactive ingredients
CYCLOMETHICONE, WATER, BUTYLENE GLYCOL, DIMETHICONE, POLYMETHYLSILSESQUIOXANE, PEG-10 DIMETHICONE, POLYBUTYLENE GLYCOL/PPG-9/1 COPOLYMER, TRIMETHYLSILOXYSILICATE, DISTEARDIMONIUM HECTORITE, GLYCERIN, XYLITOL, METHYL GLUCETH-10, SCUTELLARIA BAICALENSIS ROOT EXTRACT, ONONIS SPINOSA ROOT EXTRACT, ECTOIN, HYDROGENATED POLYDECENE, CYCLOPENTASILOXANE, TRIETHOXYCAPRYLYLSILANE, ALUMINUM HYDROXIDE, STEARIC ACID, ACRYLATES/DIMETHICONE COPOLYMER, ISOSTEARIC ACID, TRISODIUM EDTA, BHT, SYZYGIUM JAMBOS LEAF EXTRACT, TOCOPHEROL, PHENOXYETHANOL, FRAGRANCE
Questions?
1-800-906-7503
SHISEIDO AMERICA INC.
NEW YORK, N.Y. 10022
MADE IN U.S.A.
SHISEIDO DIST.
NEW YORK • PARIS • MILANO
GLO. 12627
www.shiseido.com
PRINCIPAL DISPLAY PANEL - 50mL Carton
SHISEIDO
Anti-Aging Suncare
UVA and UVB Protection
55
Ultimate
Sun Protection
Cream N
SPF 55•PA+++
For Face
VERY WATER-RESISTANT
SUNSCREEN
50mL NET WT. 2 OZ.
SHISEIDO SUNCARE ULTIMATEOctinoxate, Titanium Dioxide, and Zinc Oxide CREAM
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||