SHISEIDO AMERICA INC.
SHISEIDO PERFECT REFINING FOUNDATION
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient
Purpose
|
Active ingredients
|
Purpose
|
| OCTINOXATE |
1.9% |
Sunscreen |
| TITANIUM DIOXIDE |
4.1% |
Sunscreen |
Use
Warnings
For external use only
Do not use on damaged or broken skin
When using this product keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash occurs
Keep out of reach of children. If product is swallowed, get medical help or contact a Poison Control Center right away.
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
- children under 6 months: Ask a doctor
Inactive Ingredients
WATER, DIMETHICONE, CYCLOMETHICONE, NYLON-12, SD ALCOHOL 40-B, DIETHYLHEXYL SUCCINATE, GLYCERIN, LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE, PEG-10 DIMETHICONE, ERYTHRITOL, XYLITOL, SODIUM GLUTAMATE, POLYGLYCERYL-2 DIISOSTEARATE, PEG/PPG-36/41 DIMETHYL ETHER, SODIUM HYALURONATE, LAVANDULA ANGUSTIFOLIA (LAVENDER) OIL, PAEONIA ALBIFLORA ROOT EXTRACT, ALUMINUM HYDROXIDE, ALUMINUM DISTEARATE, ALUMINA, DISTEARDIMONIUM HECTORITE, TRIETHOXYCAPRYLYLSILANE, POLYSILICONE-2, VINYL DIMETHICONE/METHICONE SILSESQUIOXANE CROSSPOLYMER, BUTYLENE GLYCOL, TOCOPHEROL, DIPENTAERYTHRITYL HEXAHYDROXYSTEARATE, BARIUM SULFATE, SODIUM METABISULFITE, BHT, METHICONE, HYDROGEN DIMETHICONE, TETRADECENE, PHENOXYETHANOL, BENZOIC ACID, TITANIUM DIOXIDE, IRON OXIDES, ZINC OXIDE, MICA,
SHISEIDO PERFECT REFINING FOUNDATION Other information
- protect this product in this container from excessive heat and direct sun.
Questions or comments?
Call toll free 1-800-906-7503
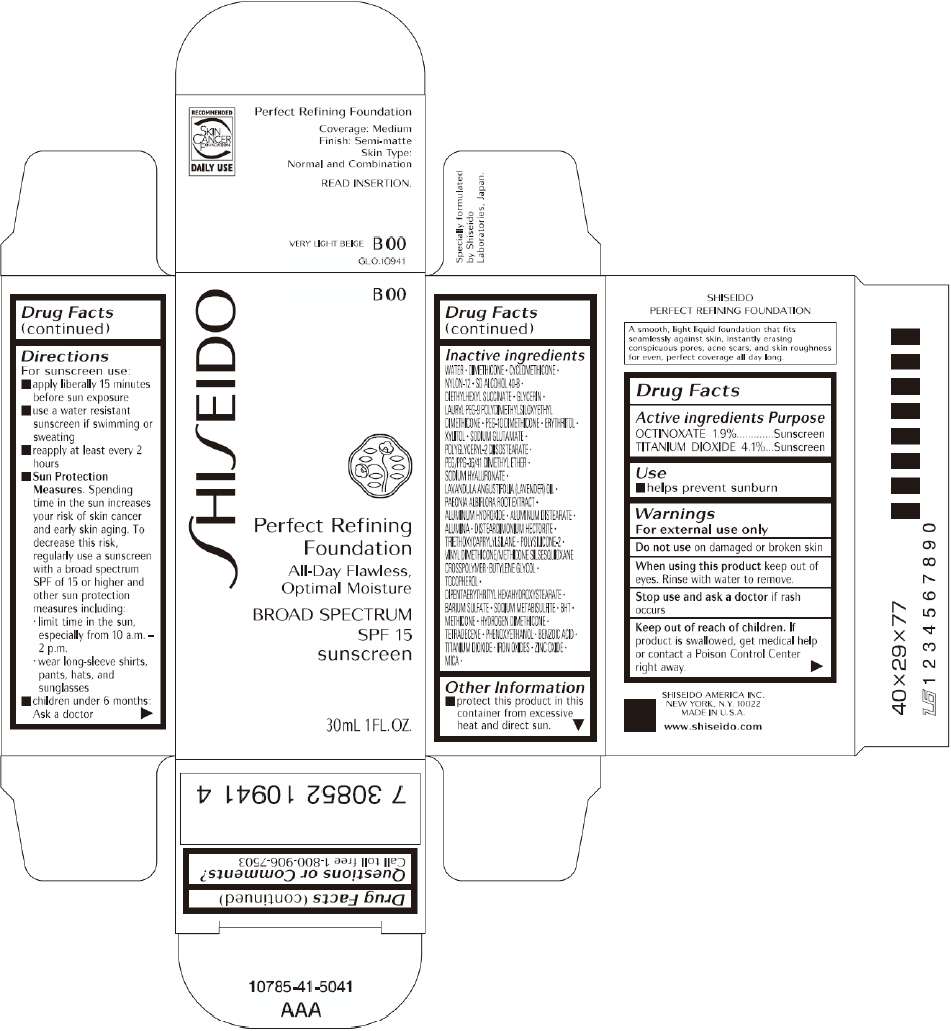
PRINCIPAL DISPLAY PANEL - 30mL B 00 Tube Carton
S
HI
S
EIDO
B 00
Perfect Refining
Foundation
All-Day Flawless,
Optimal Moisture
BROAD SPECTRUM
SPF 15
sunscreen
30mL 1FL. OZ.
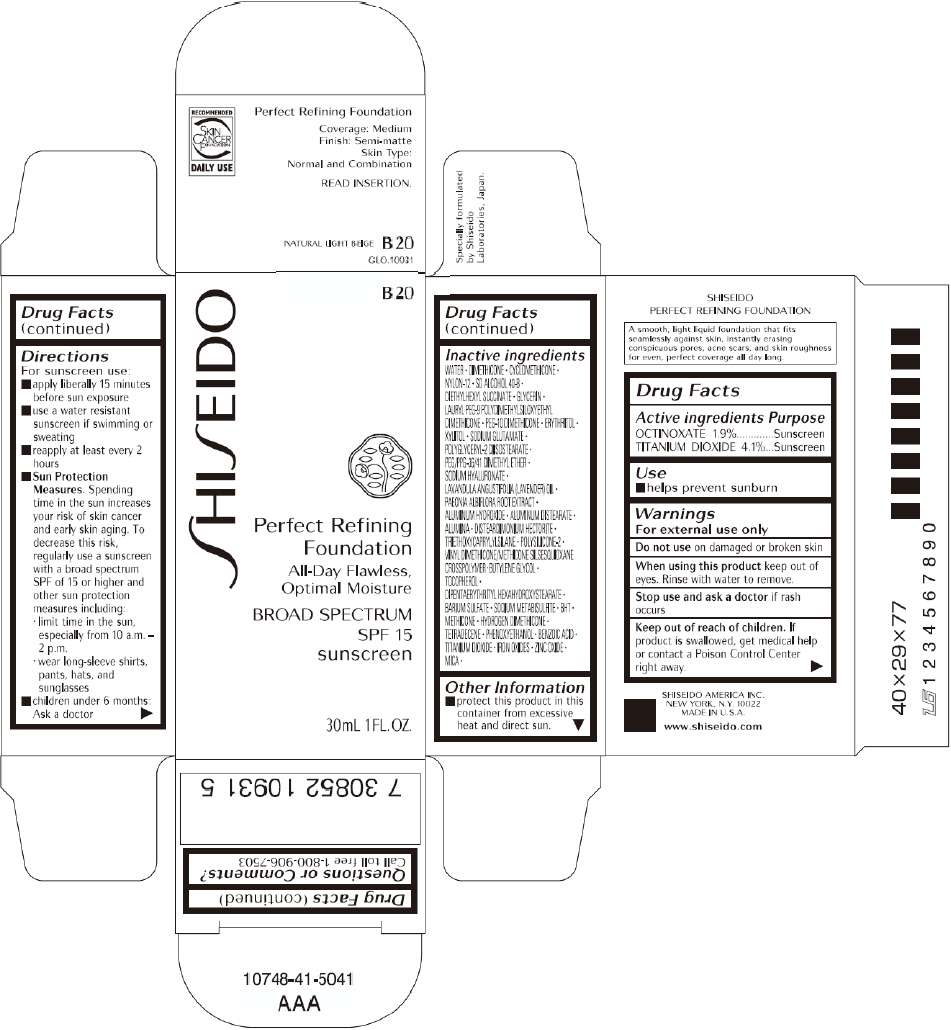
PRINCIPAL DISPLAY PANEL - 30mL B 20 Tube Carton
S
HI
S
EIDO
B 20
Perfect Refining
Foundation
All-Day Flawless,
Optimal Moisture
BROAD SPECTRUM
SPF 15
sunscreen
30mL 1FL. OZ.
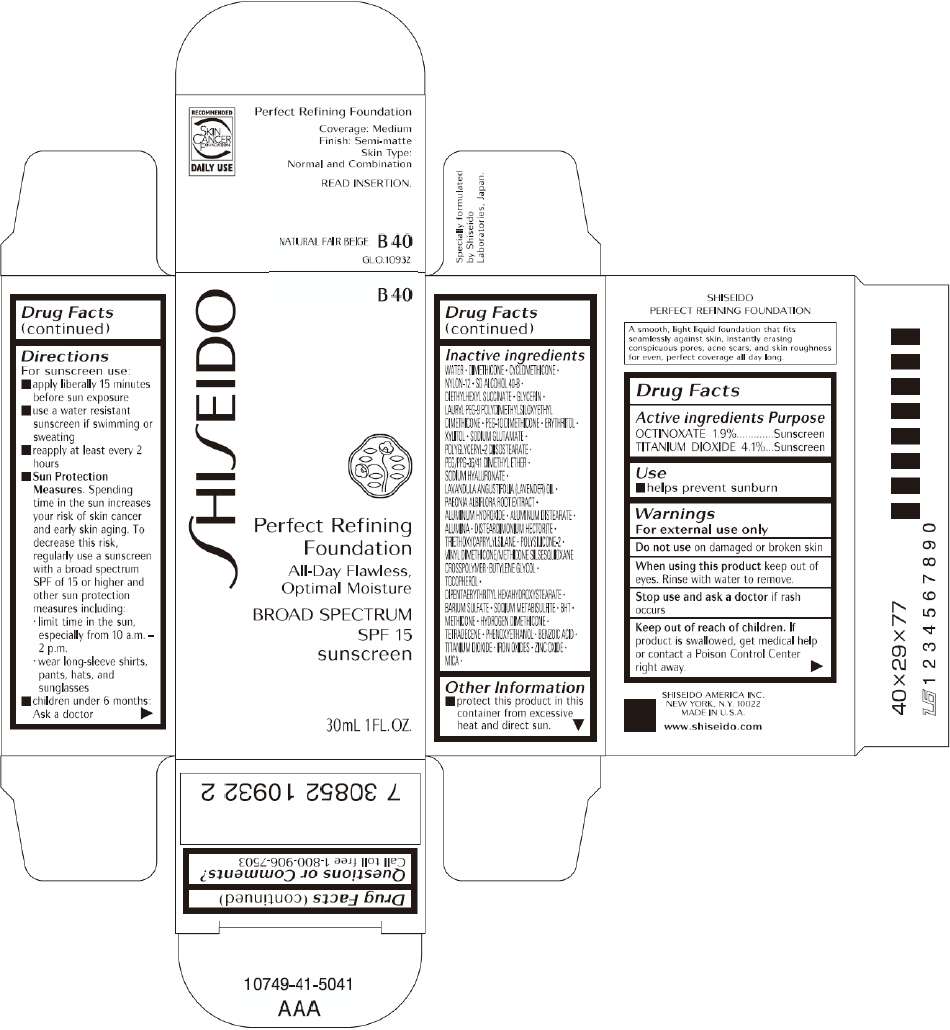
PRINCIPAL DISPLAY PANEL - 30mL B 40 Tube Carton
S
HI
S
EIDO
B 40
Perfect Refining
Foundation
All-Day Flawless,
Optimal Moisture
BROAD SPECTRUM
SPF 15
sunscreen
30mL 1FL. OZ.
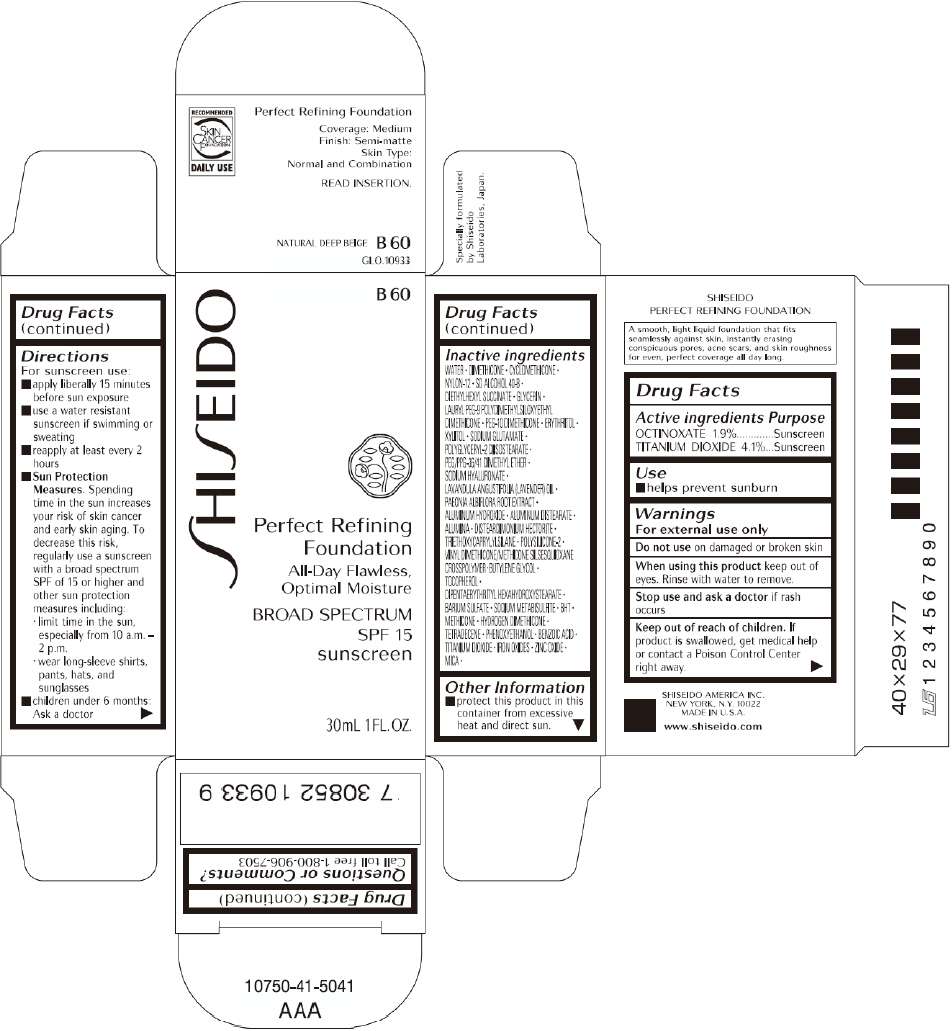
PRINCIPAL DISPLAY PANEL - 30mL B 60 Tube Carton
S
HI
S
EIDO
B 60
Perfect Refining
Foundation
All-Day Flawless,
Optimal Moisture
BROAD SPECTRUM
SPF 15
sunscreen
30mL 1FL. OZ.
PRINCIPAL DISPLAY PANEL - 30mL B 100 Tube Carton
S
HI
S
EIDO
B 100
Perfect Refining
Foundation
All-Day Flawless,
Optimal Moisture
BROAD SPECTRUM
SPF 15
sunscreen
30mL 1FL. OZ.
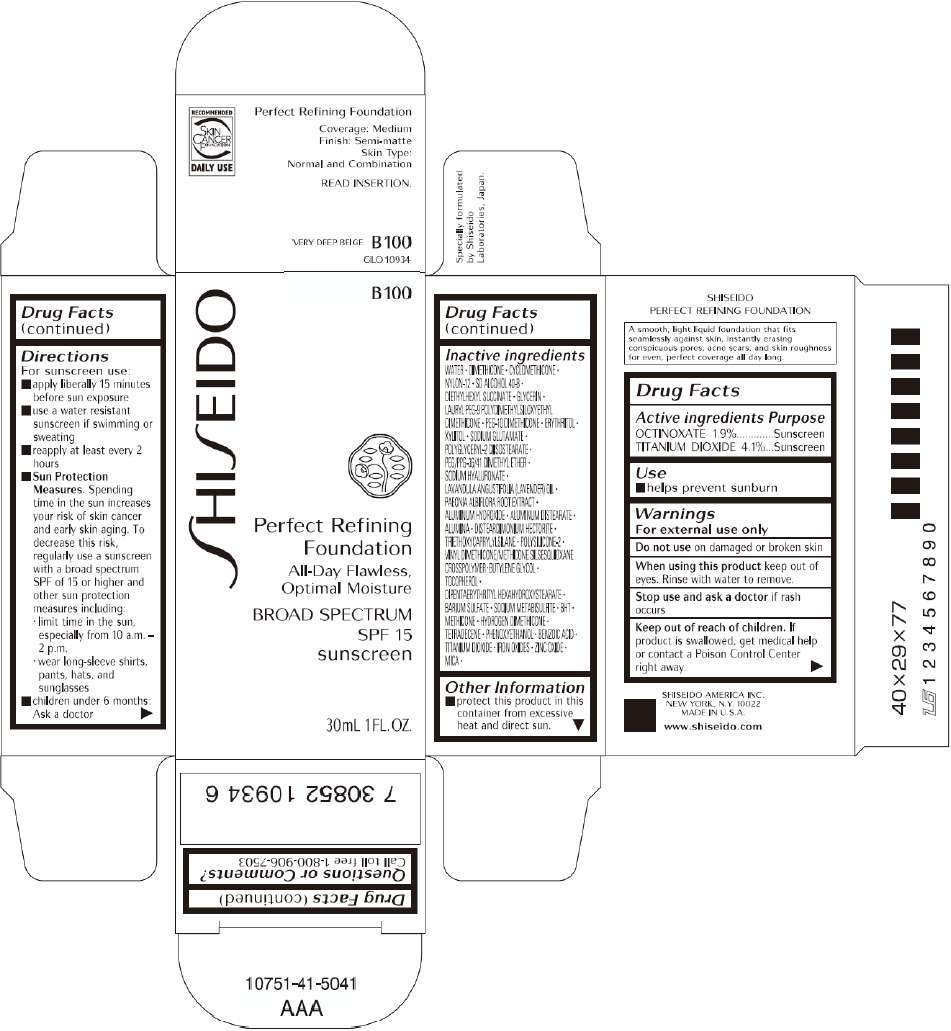
PRINCIPAL DISPLAY PANEL - 30mL I 00 Tube Carton
S
HI
S
EIDO
I 00
Perfect Refining
Foundation
All-Day Flawless,
Optimal Moisture
BROAD SPECTRUM
SPF 15
sunscreen
30mL 1FL. OZ.
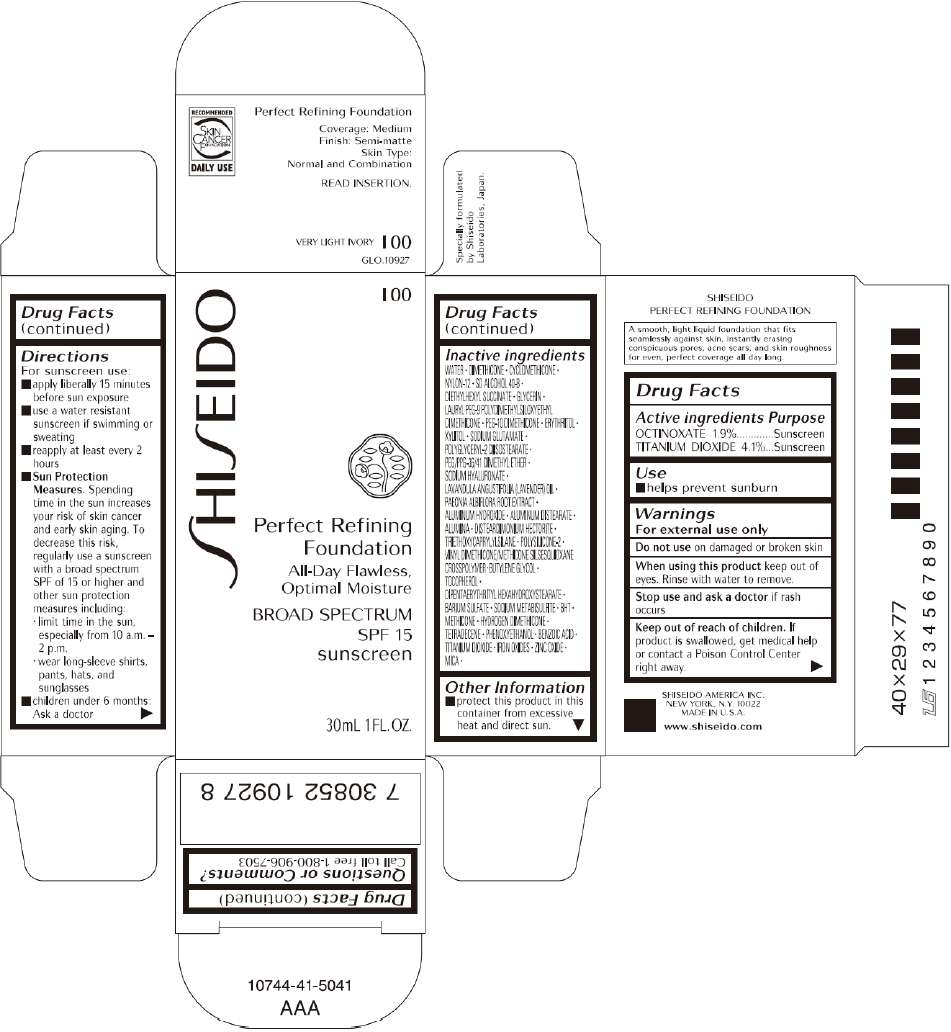
PRINCIPAL DISPLAY PANEL - 30mL I 20 Tube Carton
S
HI
S
EIDO
I 20
Perfect Refining
Foundation
All-Day Flawless,
Optimal Moisture
BROAD SPECTRUM
SPF 15
sunscreen
30mL 1FL. OZ.

PRINCIPAL DISPLAY PANEL - 30mL I 40 Tube Carton
S
HI
S
EIDO
I 40
Perfect Refining
Foundation
All-Day Flawless,
Optimal Moisture
BROAD SPECTRUM
SPF 15
sunscreen
30mL 1FL. OZ.
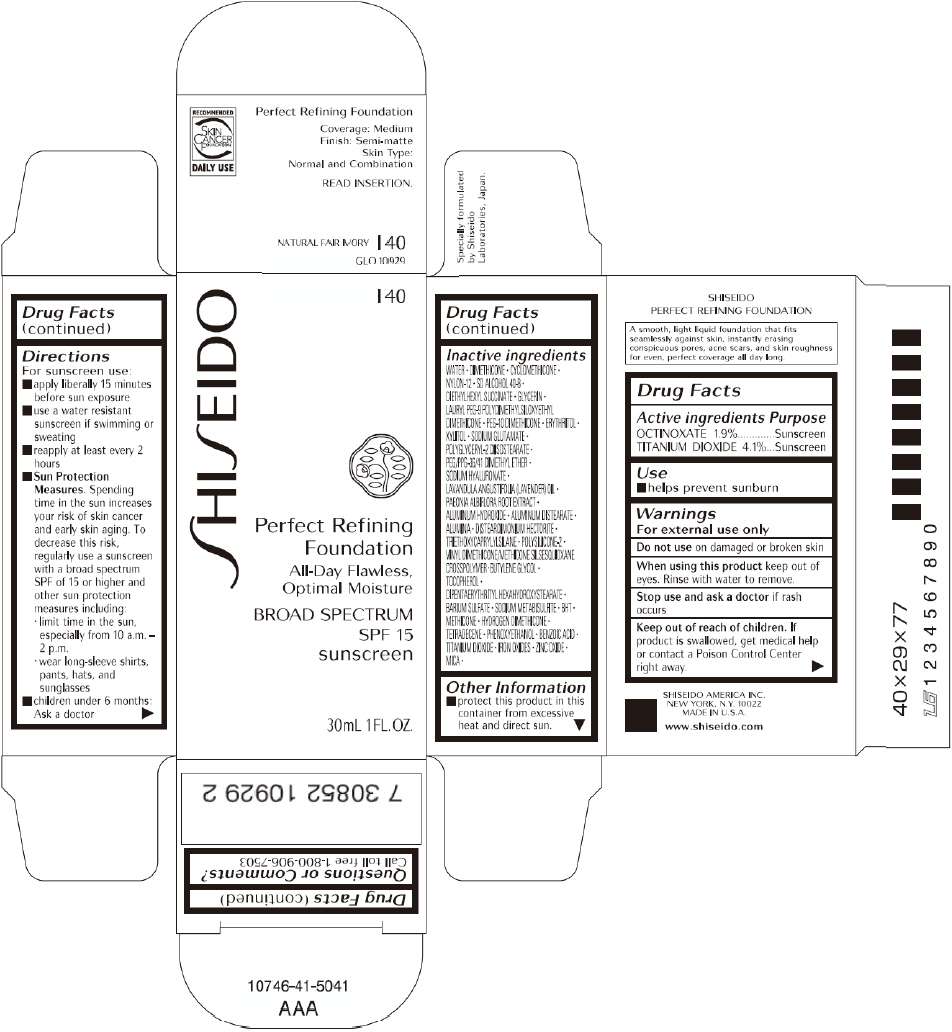
PRINCIPAL DISPLAY PANEL - 30mL I 60 Tube Carton
S
HI
S
EIDO
I 60
Perfect Refining
Foundation
All-Day Flawless,
Optimal Moisture
BROAD SPECTRUM
SPF 15
sunscreen
30mL 1FL. OZ.
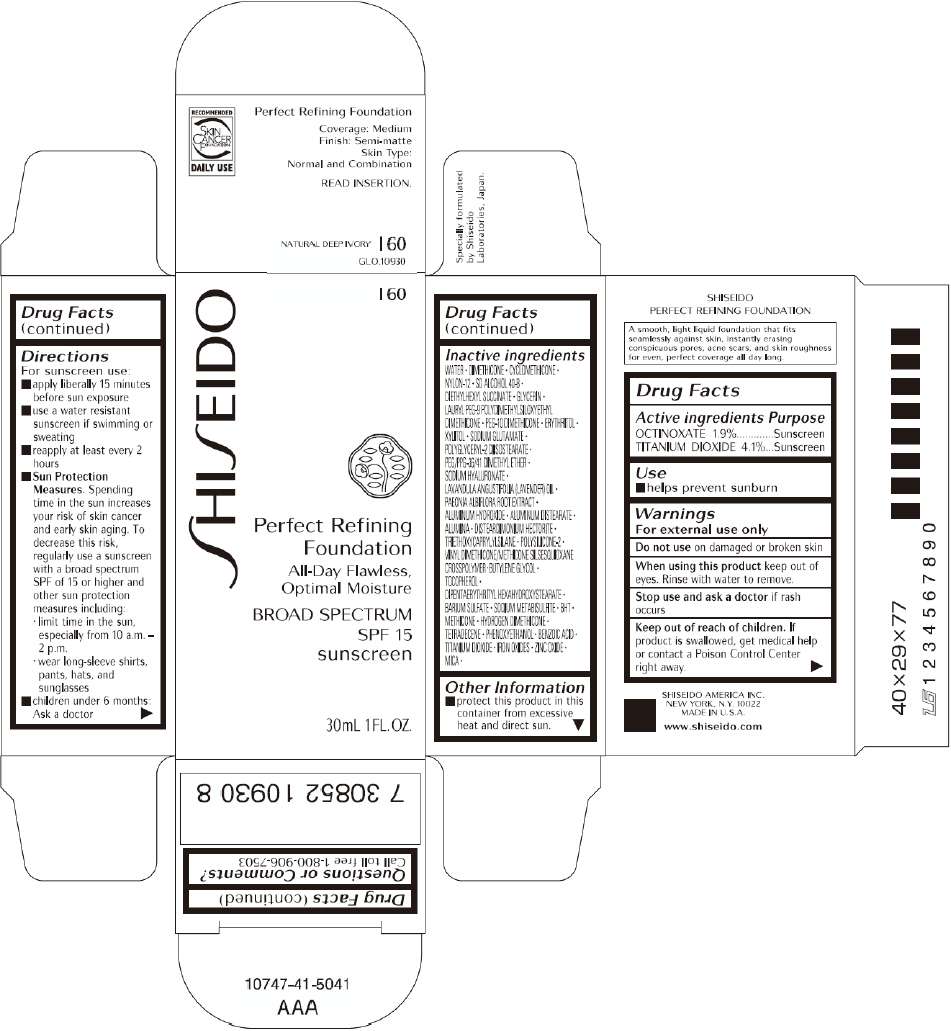
PRINCIPAL DISPLAY PANEL - 30mL I 100 Tube Carton
S
HI
S
EIDO
I 100
Perfect Refining
Foundation
All-Day Flawless,
Optimal Moisture
BROAD SPECTRUM
SPF 15
sunscreen
30mL 1FL. OZ.
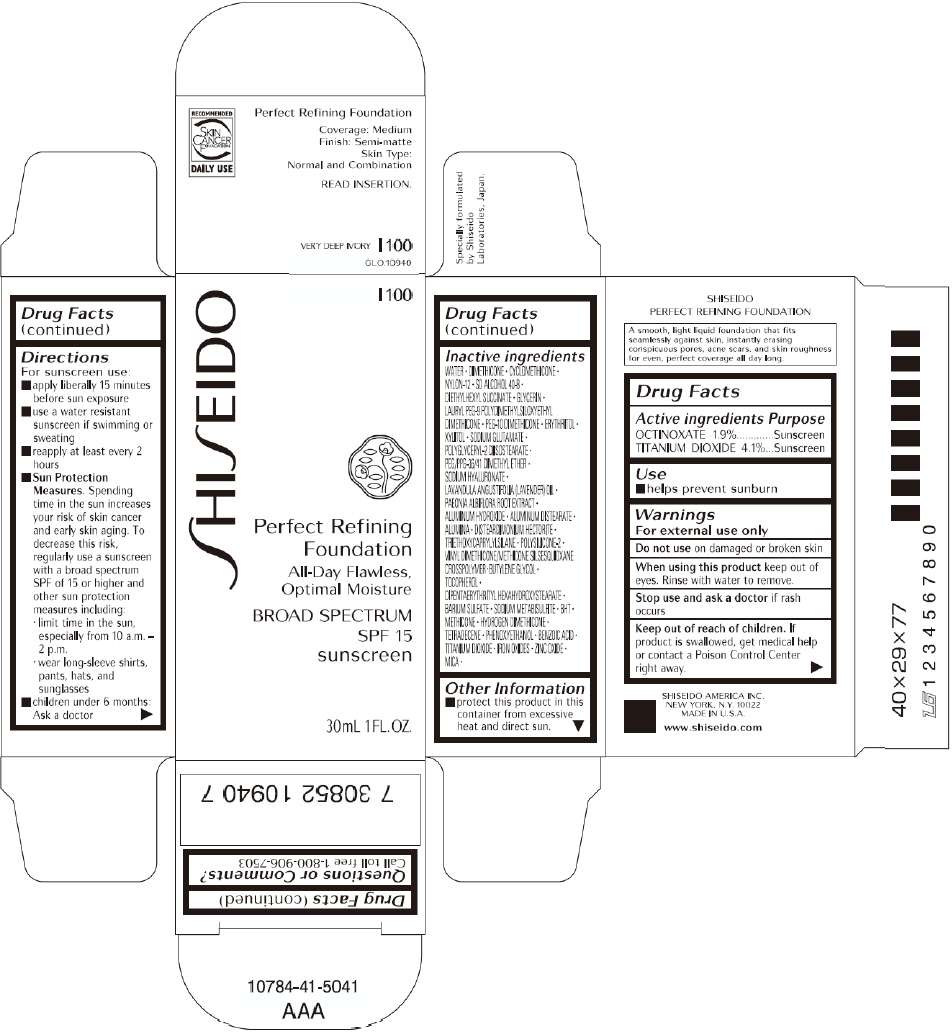
PRINCIPAL DISPLAY PANEL - 30mL O 00 Tube Carton
S
HI
S
EIDO
O 00
Perfect Refining
Foundation
All-Day Flawless,
Optimal Moisture
BROAD SPECTRUM
SPF 15
sunscreen
30mL 1FL. OZ.
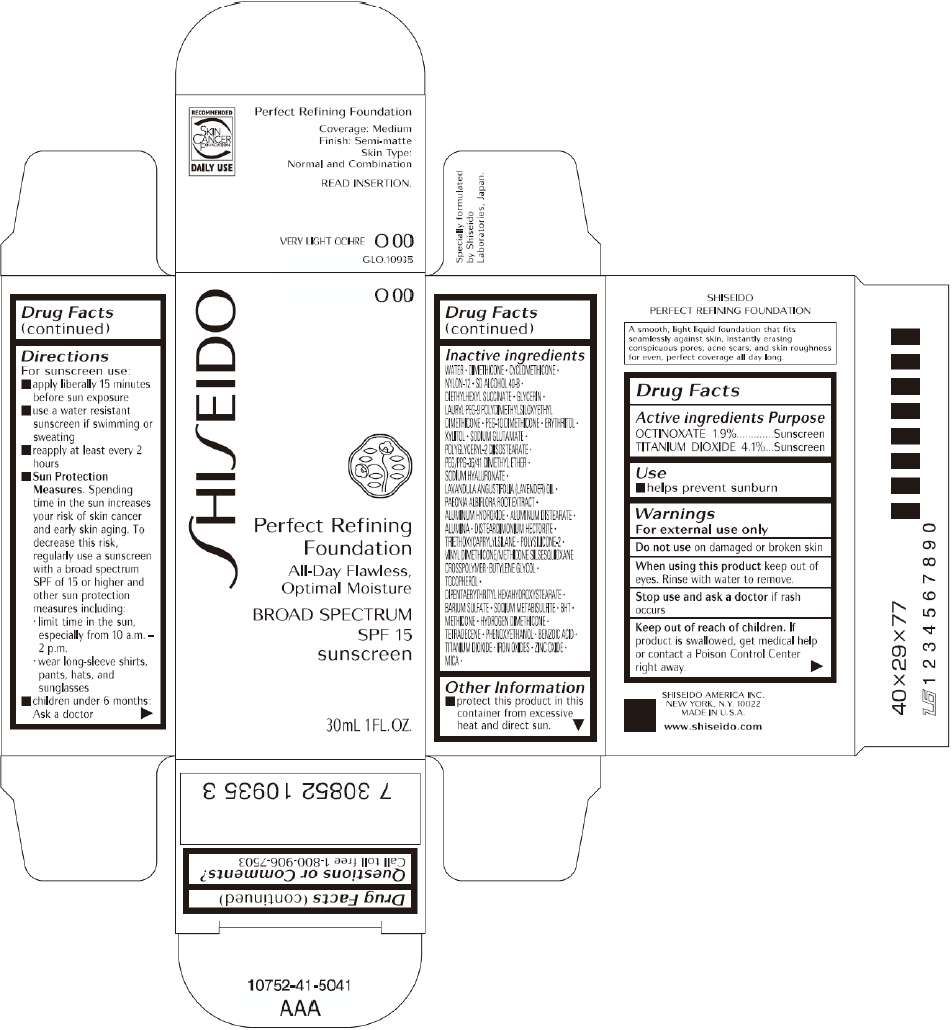
PRINCIPAL DISPLAY PANEL - 30mL O 20 Tube Carton
S
HI
S
EIDO
O 20
Perfect Refining
Foundation
All-Day Flawless,
Optimal Moisture
BROAD SPECTRUM
SPF 15
sunscreen
30mL 1FL. OZ.
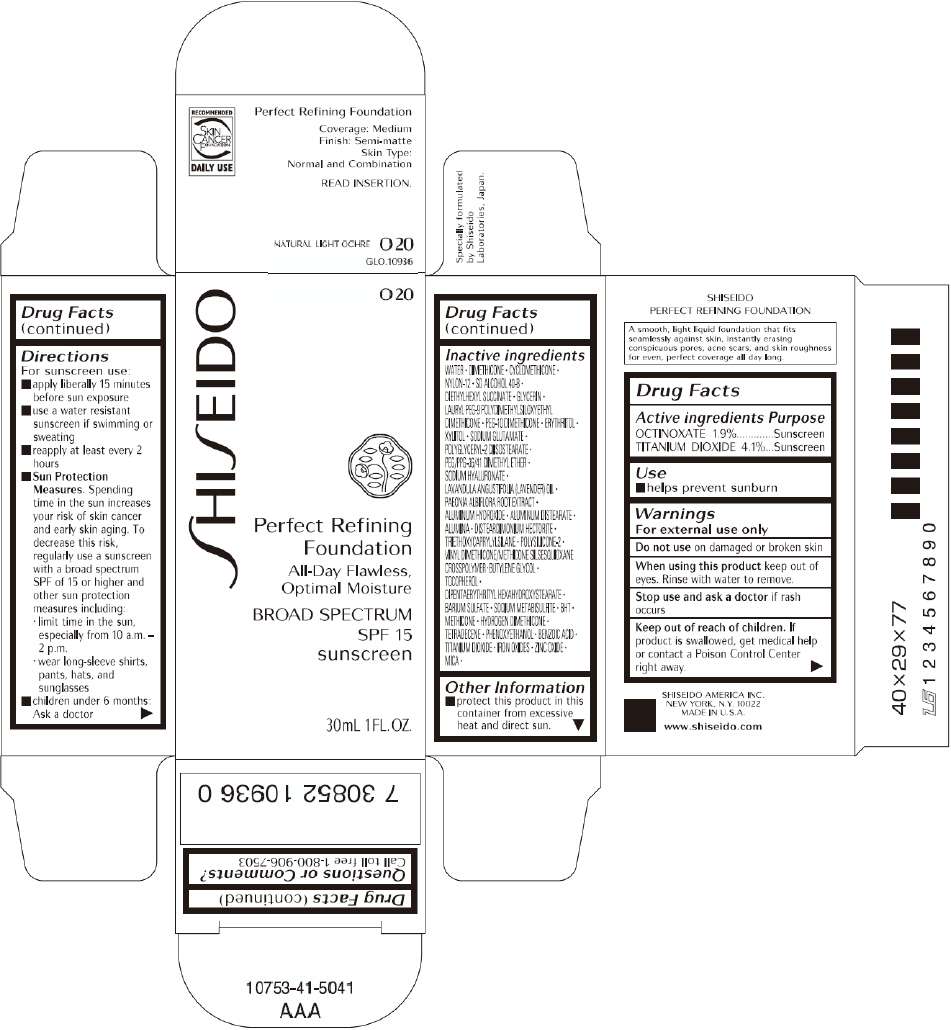
PRINCIPAL DISPLAY PANEL - 30mL O 40 Tube Carton
S
HI
S
EIDO
O 40
Perfect Refining
Foundation
All-Day Flawless,
Optimal Moisture
BROAD SPECTRUM
SPF 15
sunscreen
30mL 1FL. OZ.
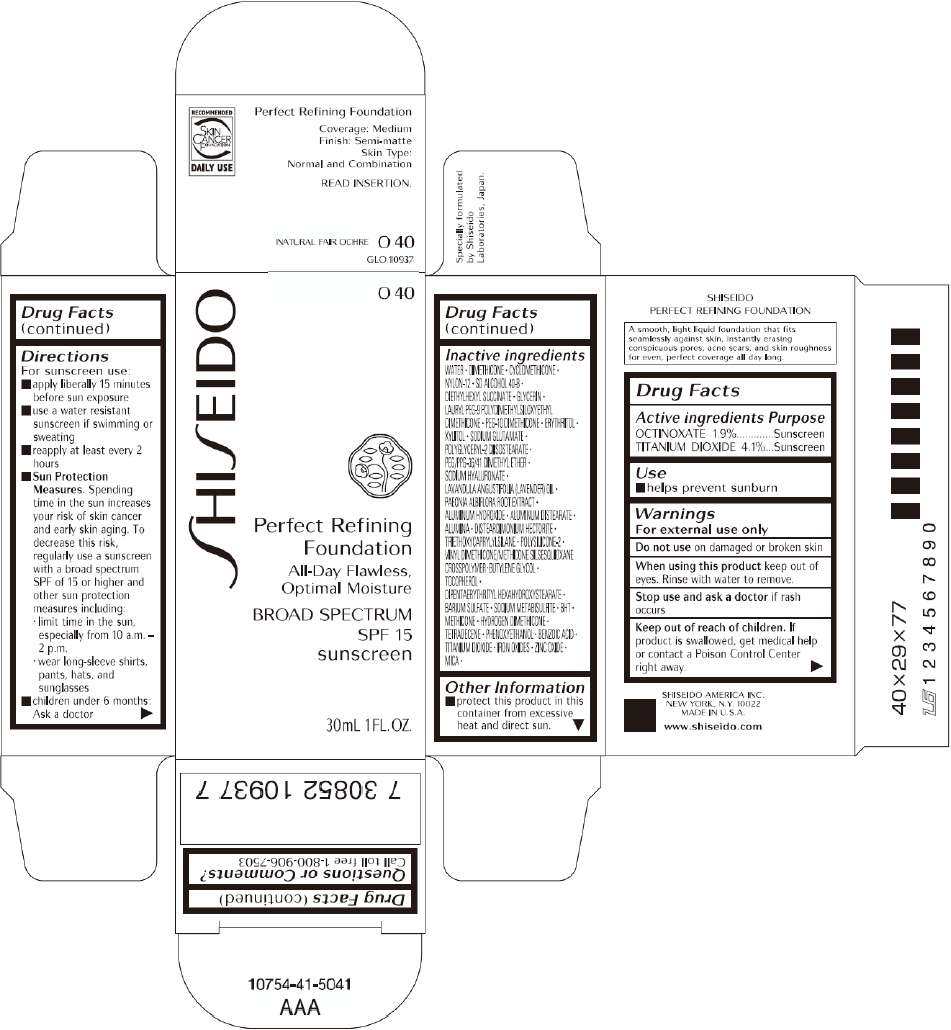
PRINCIPAL DISPLAY PANEL - 30mL O 60 Tube Carton
S
HI
S
EIDO
O 60
Perfect Refining
Foundation
All-Day Flawless,
Optimal Moisture
BROAD SPECTRUM
SPF 15
sunscreen
30mL 1FL. OZ.
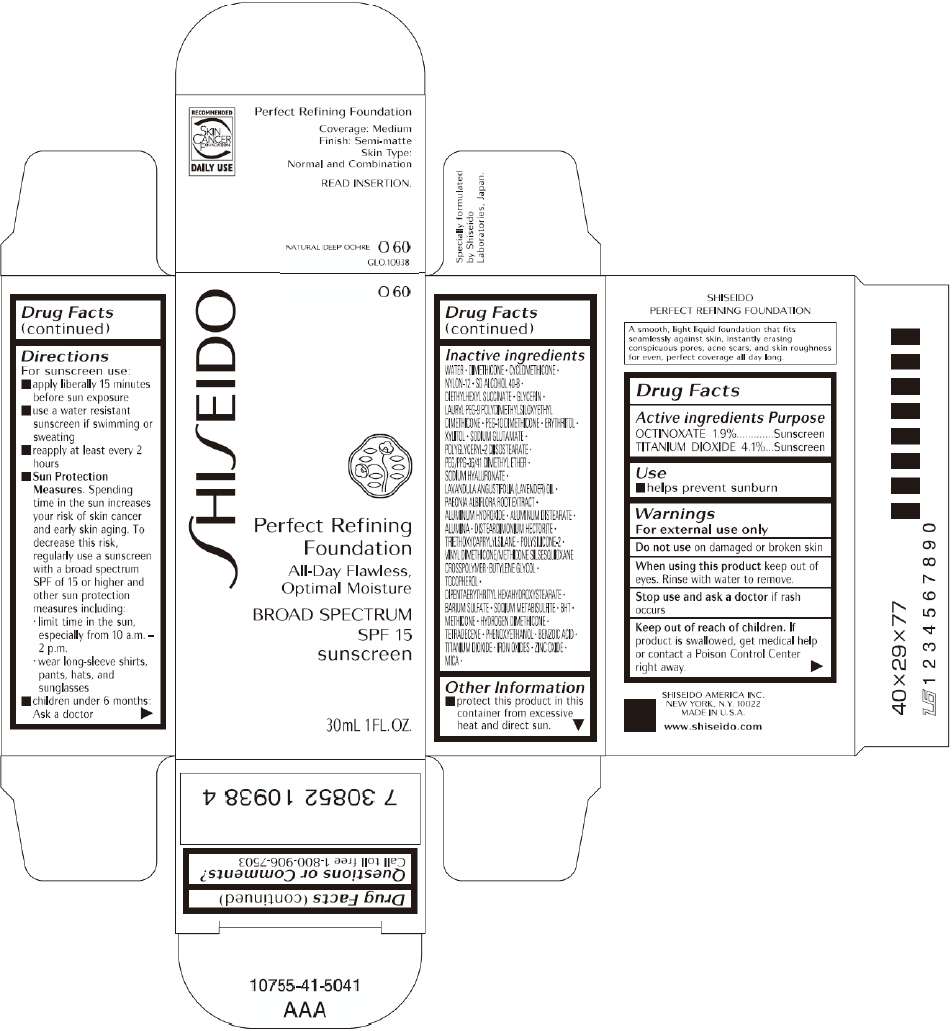
PRINCIPAL DISPLAY PANEL - 30mL O 80 Tube Carton
S
HI
S
EIDO
O 80
Perfect Refining
Foundation
All-Day Flawless,
Optimal Moisture
BROAD SPECTRUM
SPF 15
sunscreen
30mL 1FL. OZ.
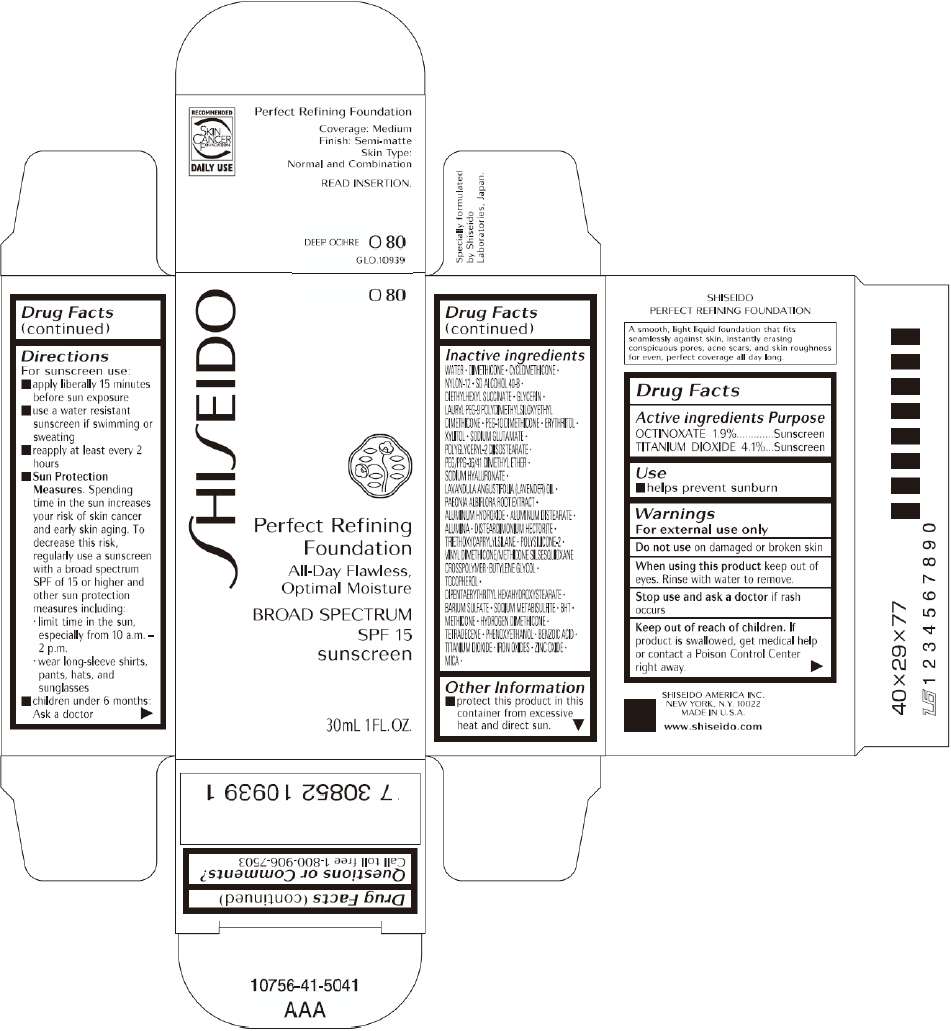
SHISEIDO PERFECT REFINING FOUNDATION
OCTINOXATE and TITANIUM DIOXIDE CREAM
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:52686-232 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.63555 g
|
|
titanium dioxide |
|
1.37145 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
33.45 in 1 TUBE |
|
|
|
2 |
NDC:52686-232-60 |
1 in 1 CARTON |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2011-02-01 |
|
|
SHISEIDO PERFECT REFINING FOUNDATION
OCTINOXATE and TITANIUM DIOXIDE CREAM
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:52686-233 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.63555 g
|
|
titanium dioxide |
|
1.37145 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
33.45 in 1 TUBE |
|
|
|
2 |
NDC:52686-233-60 |
1 in 1 CARTON |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2011-02-01 |
|
|
SHISEIDO PERFECT REFINING FOUNDATION
OCTINOXATE and TITANIUM DIOXIDE CREAM
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:52686-234 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.63555 g
|
|
titanium dioxide |
|
1.37145 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
33.45 in 1 TUBE |
|
|
|
2 |
NDC:52686-234-60 |
1 in 1 CARTON |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2011-02-01 |
|
|
SHISEIDO PERFECT REFINING FOUNDATION
OCTINOXATE and TITANIUM DIOXIDE CREAM
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:52686-235 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.63555 g
|
|
titanium dioxide |
|
1.37145 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
33.45 in 1 TUBE |
|
|
|
2 |
NDC:52686-235-60 |
1 in 1 CARTON |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2011-02-01 |
|
|
SHISEIDO PERFECT REFINING FOUNDATION
OCTINOXATE and TITANIUM DIOXIDE CREAM
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:52686-236 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.63555 g
|
|
titanium dioxide |
|
1.37145 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
33.45 in 1 TUBE |
|
|
|
2 |
NDC:52686-236-60 |
1 in 1 CARTON |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2011-02-01 |
|
|
SHISEIDO PERFECT REFINING FOUNDATION
OCTINOXATE and TITANIUM DIOXIDE CREAM
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:52686-237 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.63555 g
|
|
titanium dioxide |
|
1.37145 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
33.45 in 1 TUBE |
|
|
|
2 |
NDC:52686-237-60 |
1 in 1 CARTON |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2011-02-01 |
|
|
SHISEIDO PERFECT REFINING FOUNDATION
OCTINOXATE and TITANIUM DIOXIDE CREAM
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:52686-238 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.63555 g
|
|
titanium dioxide |
|
1.37145 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
33.45 in 1 TUBE |
|
|
|
2 |
NDC:52686-238-60 |
1 in 1 CARTON |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2011-02-01 |
|
|
SHISEIDO PERFECT REFINING FOUNDATION
OCTINOXATE and TITANIUM DIOXIDE CREAM
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:52686-239 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.63555 g
|
|
titanium dioxide |
|
1.37145 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
33.45 in 1 TUBE |
|
|
|
2 |
NDC:52686-239-60 |
1 in 1 CARTON |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2011-02-01 |
|
|
SHISEIDO PERFECT REFINING FOUNDATION
OCTINOXATE and TITANIUM DIOXIDE CREAM
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:52686-240 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.63555 g
|
|
titanium dioxide |
|
1.37145 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
33.45 in 1 TUBE |
|
|
|
2 |
NDC:52686-240-60 |
1 in 1 CARTON |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2011-02-01 |
|
|
SHISEIDO PERFECT REFINING FOUNDATION
OCTINOXATE and TITANIUM DIOXIDE CREAM
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:52686-241 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.63555 g
|
|
titanium dioxide |
|
1.37145 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
33.45 in 1 TUBE |
|
|
|
2 |
NDC:52686-241-60 |
1 in 1 CARTON |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2011-02-01 |
|
|
SHISEIDO PERFECT REFINING FOUNDATION
OCTINOXATE and TITANIUM DIOXIDE CREAM
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:52686-242 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.63555 g
|
|
titanium dioxide |
|
1.37145 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
33.45 in 1 TUBE |
|
|
|
2 |
NDC:52686-242-60 |
1 in 1 CARTON |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2011-02-01 |
|
|
SHISEIDO PERFECT REFINING FOUNDATION
OCTINOXATE and TITANIUM DIOXIDE CREAM
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:52686-243 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.63555 g
|
|
titanium dioxide |
|
1.37145 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
33.45 in 1 TUBE |
|
|
|
2 |
NDC:52686-243-60 |
1 in 1 CARTON |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2011-02-01 |
|
|
SHISEIDO PERFECT REFINING FOUNDATION
OCTINOXATE and TITANIUM DIOXIDE CREAM
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:52686-244 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.63555 g
|
|
titanium dioxide |
|
1.37145 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
33.45 in 1 TUBE |
|
|
|
2 |
NDC:52686-244-60 |
1 in 1 CARTON |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2011-02-01 |
|
|
SHISEIDO PERFECT REFINING FOUNDATION
OCTINOXATE and TITANIUM DIOXIDE CREAM
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:52686-245 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.63555 g
|
|
titanium dioxide |
|
1.37145 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
33.45 in 1 TUBE |
|
|
|
2 |
NDC:52686-245-60 |
1 in 1 CARTON |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2011-02-01 |
|
|
SHISEIDO PERFECT REFINING FOUNDATION
OCTINOXATE and TITANIUM DIOXIDE CREAM
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:52686-246 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.63555 g
|
|
titanium dioxide |
|
1.37145 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
33.45 in 1 TUBE |
|
|
|
2 |
NDC:52686-246-60 |
1 in 1 CARTON |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2011-02-01 |
|
|














