Sheer Cover Shade Adapting Mineral Foundation
Sheer Cover Shade Adapting Mineral Foundation SPF-15 (Light, Medium, Tan, Dark)
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredient
- Purpose
- Use
- Warnings
- Directions
- Inactive Ingredients
- PRINCIPAL DISPLAY PANEL - 4g Jar Label (Light)
- PRINCIPAL DISPLAY PANEL - 4g Jar Label (Medium)
- PRINCIPAL DISPLAY PANEL - 4g Jar Label (Tan)
- PRINCIPAL DISPLAY PANEL - 4g Jar Label (Dark)
FULL PRESCRIBING INFORMATION
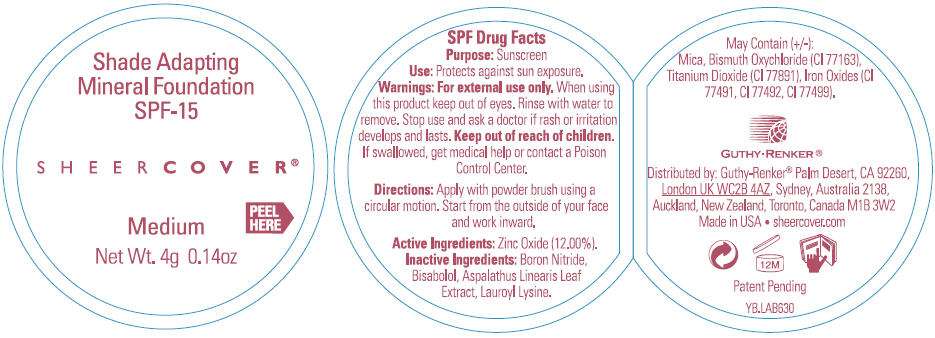
SPF Drug Facts
Active Ingredient
Zinc Oxide (12.00%)
Purpose
Sunscreen
Use
Protects against sun exposure
Warnings
For external use only. When using this product keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash or irritation develops and lasts.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Apply with powder brush using a circular motion. Start from the outside of your face and work inward.
Inactive Ingredients
boron nitride, bisabolol, aspalathus linearis leaf extract, lauroyl lysine. May contain (+/-): mica, bismuth oxychloride (CI 77163), titanium dioxide (CI 77891), iron oxides (CI 77491, CI 77492, CI 77499).
PRINCIPAL DISPLAY PANEL - 4g Jar Label (Light)
Shade Adapting
Mineral Foundation
SPF-15
SHEER COVER ®
Light
PEEL
HERE
Net Wt. 4g 0.14 oz

PRINCIPAL DISPLAY PANEL - 4g Jar Label (Medium)
Shade Adapting
Mineral Foundation
SPF-15
SHEER COVER ®
Medium
PEEL
HERE
Net Wt. 4g 0.14oz
PRINCIPAL DISPLAY PANEL - 4g Jar Label (Tan)
Shade Adapting
Mineral Foundation
SPF-15
SHEER COVER ®
Tan
PEEL
HERE
Net Wt. 4g 0.14oz

PRINCIPAL DISPLAY PANEL - 4g Jar Label (Dark)
Shade Adapting
Mineral Foundation
SPF-15
SHEER COVER ®
Dark
PEEL
HERE
Net Wt. 4g 0.14oz

Sheer Cover Shade Adapting Mineral FoundationZINC OXIDE POWDER
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
Sheer Cover Shade Adapting Mineral FoundationZINC OXIDE POWDER
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
Sheer Cover Shade Adapting Mineral FoundationZINC OXIDE POWDER
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
Sheer Cover Shade Adapting Mineral FoundationZINC OXIDE POWDER
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||