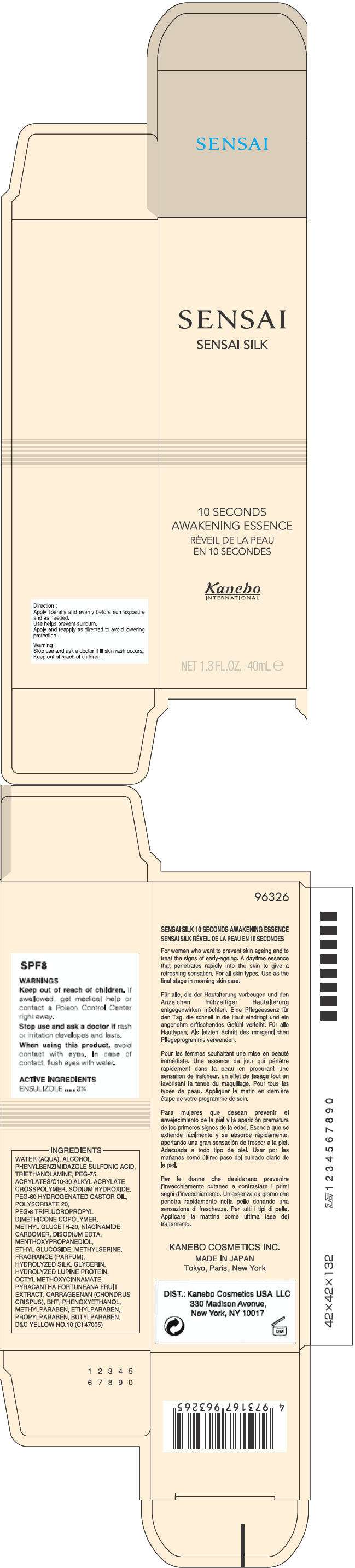
SENSAI SILK 10 SECONDS AWAKENING ESSENCE
SENSAI SENSAI SILK 10 SECONDS AWAKENING ESSENCE SPF8
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
WARNINGS
Keep out of reach of children. if swallowed, get medical help or contact a Poison Control Center right away.
Stop use and ask a doctor if rash or irritation developes and lasts.
When using this product, avoid contact with eyes. In case of contact, flush eyes with water.
ACTIVE INGREDIENTS
ENSULIZOLE..... 3%
INGREDIENTS
WATER (AQUA), ALCOHOL, PHENYLBENZIMIDAZOLE SULFONIC ACID, TRIETHANOLAMINE, PEG-75, ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER, SODIUM HYDROXIDE, PEG-60 HYDROGENATED CASTOR OIL, POLYSORBATE 20, PEG-8 TRIFLUOROPROPYL DIMETHICONE COPOLYMER, METHYL GLUCETH-20, NIACINAMIDE, CARBOMER, DISODIUM EDTA, MENTHOXYPROPANEDIOL, ETHYL GLUCOSIDE, METHYLSERINE, FRAGRANCE (PARFUM), HYDROLYZED SILK, GLYCERIN, HYDROLYZED LUPINE PROTEIN, OCTYL METHOXYCINNAMATE, PYRACANTHA FORTUNEANA FRUIT EXTRACT, CARRAGEENAN (CHONDRUS CRISPUS), BHT, PHENOXYETHANOL, METHYLPARABEN, ETHYLPARABEN, PROPYLPARABEN, BUTYLPARABEN, D&C YELLOW NO.10 (CI 47005)
Direction
Apply liberally and evenly before sun exposure and as needed.
Use helps prevent sunburn.
Apply and reapply as directed to avoid lowering protection.
Warning
Stop use and ask a doctor if
- skin rash occurs.
Keep out reach of children.
DIST.: KANEBO COSMETICS USA LLC
330 Madison Avenue, New York, NY 10017
PRINCIPAL DISPLAY PANEL - 40 mL Tube Carton
SENSAI
SENSAI SILK
10 SECONDS
AWAKENING ESSENCE
Kanebo
INTERNATIONAL
NET 1.3 FL.OZ. 40mL e
SENSAI SILK 10 SECONDS AWAKENING ESSENCEENSULIZOLE GEL
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||