SENSAI CONCEALER (BRUSH TYPE)
SENSAI CONCEALER (BRUSH TYPE) SPF 15
FULL PRESCRIBING INFORMATION: CONTENTS*
- ACTIVE INGREDIENTS
- INGREDIENTS
- Direction
- Warning
- PRINCIPAL DISPLAY PANEL - 2.6mL Carton
- PRINCIPAL DISPLAY PANEL - 2.6mL Carton
- PRINCIPAL DISPLAY PANEL - 2.6mL Carton
FULL PRESCRIBING INFORMATION
ACTIVE INGREDIENTS
Purpose
TITANIUM DIOXIDE 13.0% Sunscreen
INGREDIENTS
WATER (AQUA), TITANIUM DIOXIDE, CYCLOMETHICONE, DIPROPYLENE GLYCOL, MINERAL OIL (PARAFFINUM LIQUIDUM), PHYTOSTERYL/ISOSTEARYL/CETYL/STEARYL/BEHENYL DIMER DILINOLEATE, PROPYLENE GLYCOL DICAPRYLATE, SORBITAN ISOSTEARATE, DIISOSTEARYL MALATE, MALTITOL, PETROLATUM, POLYMETHYLSILSESQUIOXANE, TRIBEHENIN, BUTYROSPERMUM PARKII (SHEA BUTTER), LAUROYL LYSINE, SQUALANE, HYDROGENATED LECITHIN, STEARYL GLYCYRRHETINATE, TOCOPHERYL NICOTINATE, FRAGRANCE (PARFUM), ALCOHOL, HYDROLYZED SILK, METHICONE, HYDROLYZED RICE EXTRACT, TOCOPHEROL, OCTYLDODECYL LACTATE, STEARIC ACID, MYRISTIC ACID, SILK POWDER (SERICA), PHENOXYETHANOL, SORBIC ACID, METHYLPARABEN, ETHYLPARABEN, BUTYLPARABEN, [MAY CONTAIN(+/-): IRON OXIDES (CI 77491), IRON OXIDES (CI 77492), IRON OXIDES (CI 77499), MICA (CI 77019), SYNTHETIC FLUORPHLOGOPITE, TALC]
Direction
Apply liberally and evenly before sun exposure and as needed.
Use helps prevent sunburn.
Apply and reapply as directed to avoid lowering protection.
Warning
Stop use and ask a doctor if
- skin rash occurs.
Keep out of reach of children.
WARNING: Keep out of the eyes. Discontinue use if irritation appears.
DIST.: KANEBO COSMETICS USA LLC
330 Madison Avenue, New York, NY 10017
PRINCIPAL DISPLAY PANEL - 2.6mL Carton
SENSAI
SPF 15
CONCEALER
(BRUSH TYPE)
Kanebo
INTERNATIONAL
NET WT. 0.09 OZ. 2.6mL
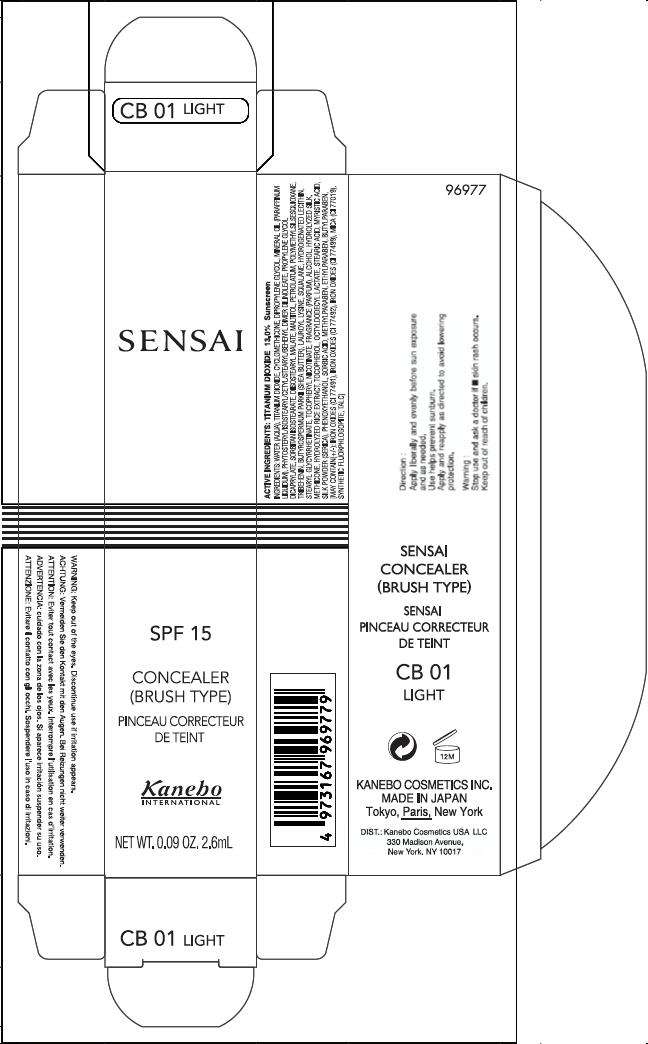
PRINCIPAL DISPLAY PANEL - 2.6mL Carton
SENSAI
SPF 15
CONCEALER
(BRUSH TYPE)
Kanebo
INTERNATIONAL
NET WT. 0.09 OZ. 2.6mL

PRINCIPAL DISPLAY PANEL - 2.6mL Carton
SENSAI
SPF 15
CONCEALER
(BRUSH TYPE)
Kanebo
INTERNATIONAL
NET WT. 0.09 OZ. 2.6mL

SENSAI CONCEALER (BRUSH TYPE)TITANIUM DIOXIDE LIQUID
| |||||||||||||||||||||||||||||||||||||||||||||||||
SENSAI CONCEALER (BRUSH TYPE)TITANIUM DIOXIDE LIQUID
| |||||||||||||||||||||||||||||||||||||||||||||||||
SENSAI CONCEALER (BRUSH TYPE)TITANIUM DIOXIDE LIQUID
| |||||||||||||||||||||||||||||||||||||||||||||||||