Senna Plus
Senna Plus Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- Senna Plus Uses
- Warnings
- Directions
- Other Information
- Inactive ingredients
- Package/Label Principal Display Panel
FULL PRESCRIBING INFORMATION
Active ingredient
Sennosides from Senna Concentrate 8.6mg
Docusate Sodium 50mg
Purpose
Laxative & Stool Softener
Senna Plus Uses
For the short term relief of constipation. Produces bowel movement in 6 to 12 hours.
Warnings
Do not use ° for longer than one week ° when abdominal pain, nausea or vomiting are present.
Ask a doctor or pharmacist before use if you have noticed a sudden change in bowel habits that lasts over a period of two weeks.
Stop use and ask a doctor if ° you have rectal bleeding ° you fail to have a bowel movement after use of this product, this may indicate a serious condition.
If pregnant or breast-feeding, ask a health care professional before use.
KEEP OUT OF REACH OF CHILDREN
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
° Take preferably at bedtime or as directed by a doctor ° if you do not have a comfortable bowel movement by the second day, increase dose by one table (do not exceed the maximum dosage) or decrease dose until you are comfortable.
Adults & children over 12 years: 2 tablets once a day (maximum dosage, 4 tablets twice a day)
Children 6 to under 12 years: 1 tablet once a day (maximum dosage, 2 tablets twice a day)
Children 2 to under 6 years: 1.2 tablet once a day (maximum dosage, 1 tablet twice a day)
Children under 2 years: do not use
Other Information
Store at room temperature, USP.
Inactive ingredients
Croscarmellose Sodium, Dicalcium Phosphate, FD&C Yellow No. 5, FD&C Yellow No. 6, Hypromellose, Magnesium Silicate, Magnesium Stearate, Microcrystalline Cellulose, Mineral Oil, Polyethylene Glycol, Sodium Benzoate, Starch, Stearic Acid and Titanium Dioxide.
Distributed by: Plus Pharma
Commack, NY 11725
Repackaged by: Rebel Distributors Corp
Thousand Oaks, CA 91320
Package/Label Principal Display Panel
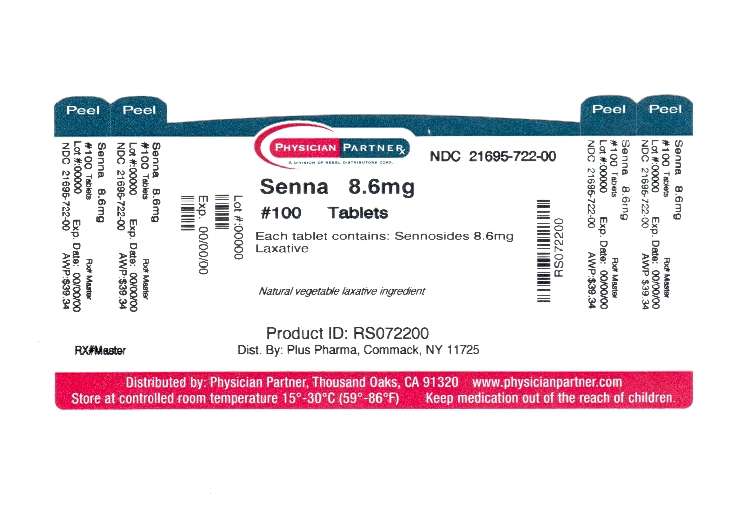
Senna PlusSenna Plus TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||