Senna-Lax
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- Keep Out of Reach of Children
- Senna-Lax Uses
- Warnings
- Directions
- Inactive ingredients
- Storage
- Questions?
- Other Information
- Package/Label Principal Display Panel
FULL PRESCRIBING INFORMATION
Active ingredient
Drug Facts
Active Ingredient (in each tablet)
Sennosides 8.6 mg
Purpose
Laxative
Keep Out of Reach of Children
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control
Center right away.
Senna-Lax Uses
■ relieves occasional constipation (irregularity) and generally produces a bowel movement
in 6-12 hours
Warnings
Do not use laxative products for longer than 1 week unless directed by a doctor
Ask a doctor before use if you have stomach pain, nausea, vomiting or if you have noticed a sudden change in bowel habits that continues over a period of 2 weeks.
Stop use and ask a doctor if you have rectal bleeding or fail to have a bowel movement after the use of a laxative. These may indicate a serious condition.
This product is intended for institutional use only.
If pregnant or breast-feeding, ask a health professional before use.
Directions
■ take preferably at bedtime or as directed by a doctor
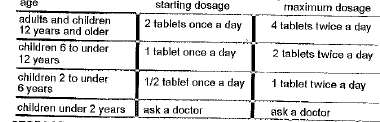
Inactive ingredients
Acacia Gum, Calcium Carbonate, Croscarmellose Sodium, Dextrose, Hypromellose, Magnesium Stearate, Maltodextrin, Microcrystalline Cellulose, Propylene Glycol, Silicon Dioxide, Stearic Acid, Triacetin.
Storage
Store at Room Temperature.
Questions?
Adverse Drug Event Call: (800) 616-2471
Other Information
Each tablet contains: Calcium 20 mg
Tamper Evident: Do not use if sealed blister units are broken or damaged
Dist. By Major Pharmaceuticals
31778 Enterprise Drive
Livonia, MI 48150
MFGD By Gemini Pharm., NY 11725
Senna-Lax 8.6 mg
Standardized Senna Concentrate
Sennosides 10 Tablets
Repackaged by Cardinal Health
Zanesville, OH 43701
L35628241010
Package/Label Principal Display Panel
SENNA- LAX 8.6 MG
Standardized Senna Concentrate
Sennosides
10 TABLETS

Senna-LaxSennosides TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||