Sei Bella
Sei Bella Daytime Delivery Creme Content of Label
FULL PRESCRIBING INFORMATION
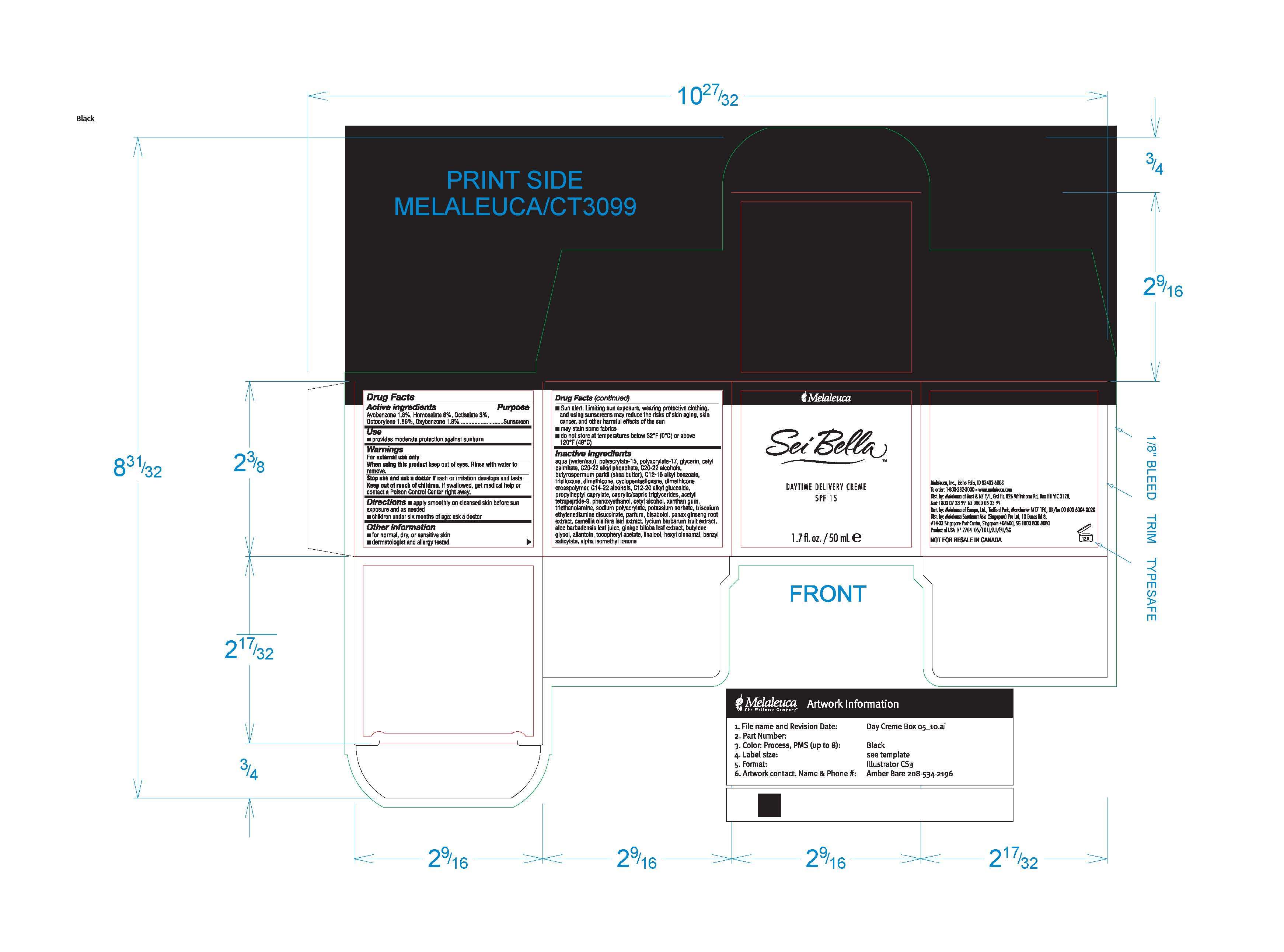
Active ingredient
Active ingredients
Avobenzone 1.8%, Homosalate 6%, Octisalate, 3%, Octocrylene 1.86%, Oxybenzone 1.8%
Purpose
Purpose
Sunscreen
Uses
Use
- provides moderate protection against sunburn
Warnings
For external use only
When using this product keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash or irritation develops and lasts
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- apply smoothly on cleansed skin before sun exposure and as needed
- children under six months of age: ask a doctor
- dermatologist and allergy tested
Other information
- Sun alert: Limiting sun exposure, wearing protective clothing, and using sunscreens may reduce the risks of skin aging, skin cancer, and other harmful effects of the sun
- may stain some fabrics
Other information
- do not store at temperatures below 32°F (0°C) or above 120°F (49°C)
Inactive ingredients
aqua (water/eau), polyacrylate-15, polyacrylate-17, glycerin, cetyl palmitate, C20-22 alkyl phosphate, C20-22 alcohols, butyrospermum parkii (shea butter), C12-15 alkyl benzoate, trisiloxane, dimethicone, cyclopentasiloxane, dimethicone crosspolymer, C14-22 alcohols, C12-20 alkyl glucoside, propylheptyl caprylate, caprylic/capric triglycerides, acetyl tetrapeptide-9, phenoxyethanol, cetyl alcohol, xanthan gum, triethanolamine, sodium polyacrylate, potassium sorbate, trisodium ethylenediamine disuccinate, parfum, bisabolol, panax ginseng root extract, camellia oleifera leaf extract, lycium barbarum fruit extract, aloe barbadensis leaf juice, ginkgo biloba leaf extract, butylene glycol, allantoin, tocopheryl acetate, linalool, hexyl cinnamal, benzyl salicylate, alpha isomethyl ionone
Sei BellaAvobenzone, Homosalate, Octisalate, Octocrylene, Oxybenzone CREAM
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||