Secret Outlast Invisible
Procter & Gamble Manufacturing Company
Secret Outlast™ Invisible Unscented
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- Use
- Warnings
- Directions
- Inactive ingredients
- Questions?
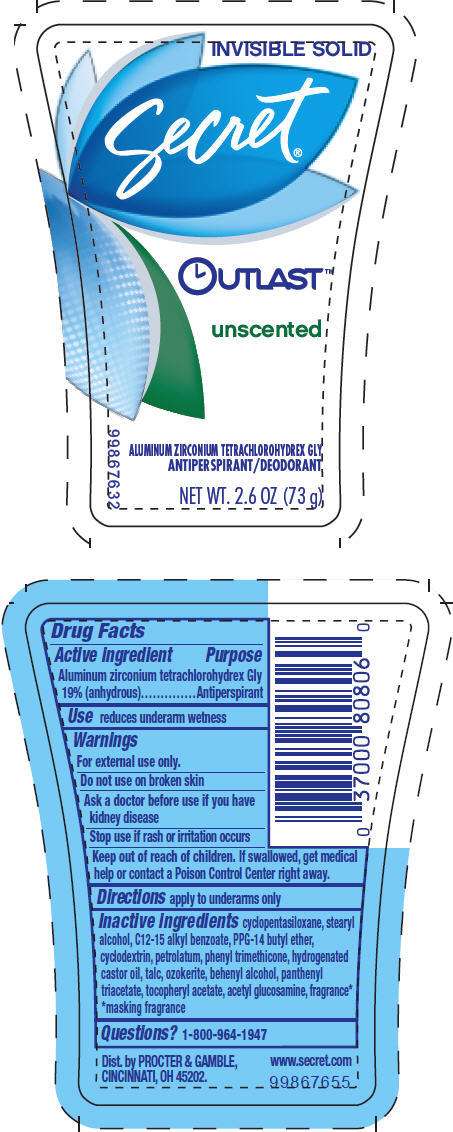
- PRINCIPAL DISPLAY PANEL - 73 g Canister Label
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient
Aluminum zirconium tetrachlorohydrex Gly 19% (anhydrous)
Purpose
Antiperspirant
Use
reduces underarm wetness
Warnings
For external use only.
Do not use on broken skin
Ask a doctor before use if you have kidney disease
Stop use if rash or irritation occurs
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
apply to underarms only
Inactive ingredients
cyclopentasiloxane, stearyl alcohol, C12-15 alkyl benzoate, PPG-14 butyl ether, cyclodextrin, petrolatum, phenyl trimethicone, hydrogenated castor oil, talc, ozokerite, behenyl alcohol, panthenyl triacetate, tocopheryl acetate, acetyl glucosamine, fragrance* *masking fragrance
Questions?
1-800-964-1947
Dist. by PROCTER & GAMBLE,
CINCINNATI, OH 45202.
PRINCIPAL DISPLAY PANEL - 73 g Canister Label
INVISIBLE SOLID
Secret ®
OUTLAST™
unscented
ALUMINUM ZIRCONIUM TETRACHLOROHYDREX GLY
ANTIPERSPIRANT/DEODORANT
NET WT. 2.6 OZ (73 g)
99867632
Secret Outlast InvisibleALUMINUM ZIRCONIUM TETRACHLOROHYDREX GLY STICK
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||