Scrub
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- Scrub Uses
- Warnings
- Directions
- Scrub Other information
- Inactive ingredients
- Questions
- Principal Display Panel
- Principal Display Panel
- Principal Display Panel
FULL PRESCRIBING INFORMATION
Active ingredient
Povidone-Iodine 7.5%
Purpose
Antiseptic
Scrub Uses
- for preparation of the skin prior to surgery
- helps to reduce bacteria that potentially can cause skin infection
- for handwashing to reduce bacteria on the skin
- significantly reduces the number of microorganisms on the hands and forearms prior to surgery or patient care
Warnings
For external use only
Do not use
- in the eyes
- if you are allergic to iodine or any of the other ingredients in the product
When using this product
- prolonged exposure to wet solution may cause irritation or, rarely, severe skin reactions
- in pre-operative prepping, avoid “pooling” beneath the patient
Stop use and ask a doctor if you have
- irritation and redness develop
- if condition persists for more than 72 hours
Keep out of reach of children.
If swallowed, get medical help or contact a Poison control center right away.
Directions
Surgical hand scrub:
- Clean under nails with a nail pick. Nails should be maintained with a 1 millimeter free edge.
- Wet hands and forearms
- Apply 5 milliliters (teaspoonful) or palmful to hands and forearms
- Scrub thoroughly for about 5 minutes paying particular attention to the nails, cuticles, and interdigital spaces
- Use a sterile scrub brush, if desired
- Rinse and repeat scrub
Patient Pre-operative Skin Preparation:
- Prior to surgery, wet skin with water
- Apply scrub to the operative site
- Scrub thoroughly for about 5 minutes
- Rinse using sterile gauze or towel, or remove with 70 percent alcohol
- Follow with application of Medline Prep solution and allow to dry
Scrub Other information
Protect from freezing. Avoid excessive heat.
Inactive ingredients
Citric Acid, Cocamide DEA, Disodium Phosphate, Sodium Hydroxide, Sodium Lauryl Sulfate, Water
Questions
1-800-MEDLINE
Principal Display Panel

NDC 53329-938-04
PVP Scrub Solution
Antiseptic
Povidone-Iodine 7.5% Sudsing Skin Cleanser
REF 55113
latexfree Do not reuse
www.medline.com
Manufactured for: Medline Industries, Inc.,
Mundelein, IL 60060 USA Made in USA
To Order Call: 1-800-MEDLINE
4 FL OZ (118 mL)
Principal Display Panel
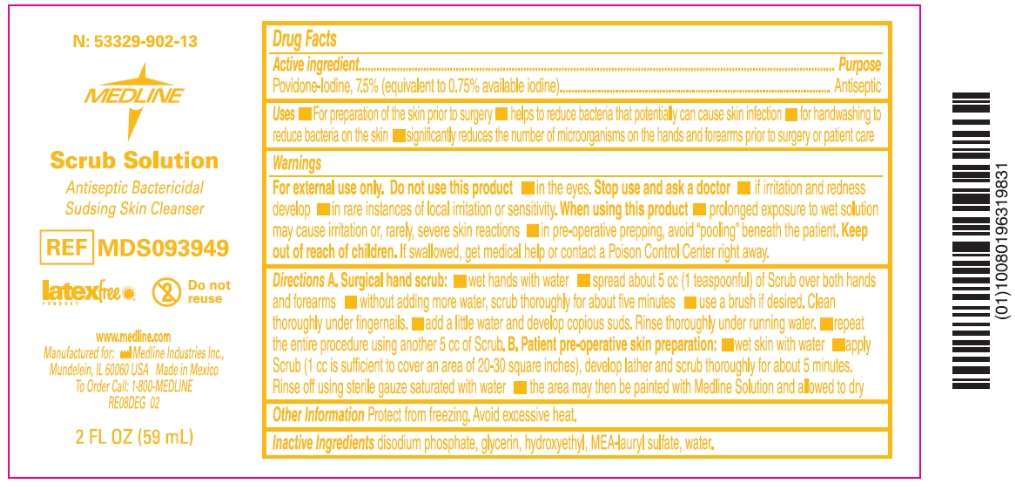
N: 53329-902-13
Scrub Solution
Antiseptic Bactericidal
Sudsing Skin Cleanser
REF MDS093949
latex free Do not reuse
www.medline.com
Manufactured for: Medline Industries, Inc.,
Mundelein, IL 60060 USA Made in USA
To Order Call: 1-800-MEDLINE
2 FL OZ (59 mL)
Principal Display Panel

NDC 53329-905-04
Scrub Solution
with Spray Bottle
Povidone Iodine USP, 7.5% Topical Solution
REF MDS093935
Topical Antiseptic
4 FL OZ (118 ml)
ScrubPovidone-Iodine SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
ScrubPovidone-Iodine SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
ScrubPovidone-Iodine SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||