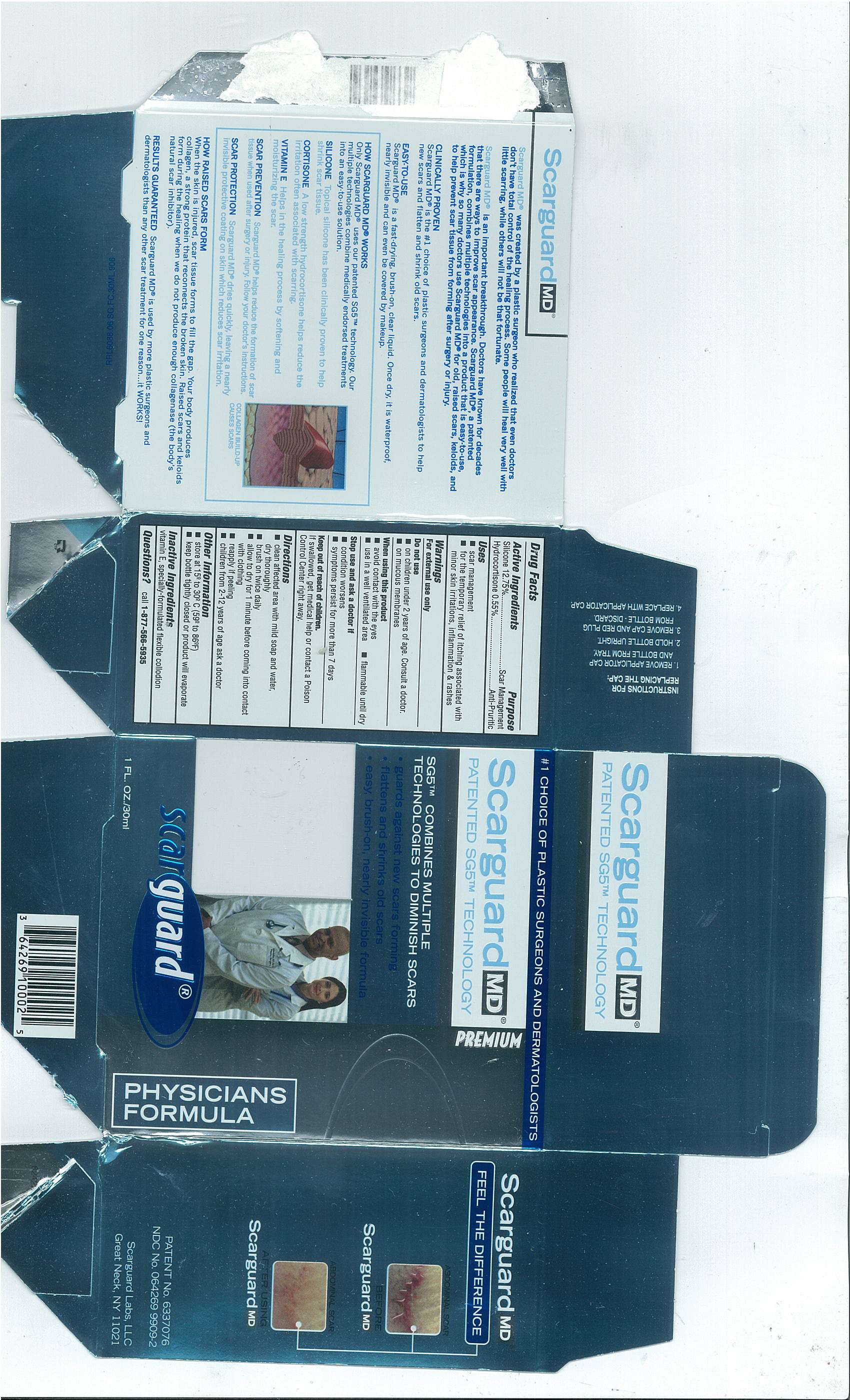
Scarguard MD Physicians Formula
Scarguard Labs, LLC
Scarguard Labs, LLC
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredients
- Purpose
- Scarguard MD Physicians Formula Uses
- Warnings
- Do not use
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children
- Directions
- Other Information
- Inactive Ingredients
- Questions?
- Carton 30mL
FULL PRESCRIBING INFORMATION
Active Ingredients
Silicone 12.75%
Hydrocortisone 0.55%
Purpose
Scar Management
Anti-Pruritic
Scarguard MD Physicians Formula Uses
- scar management
- for the temporary relief of itching associated with minor skin irritations, inflammation and rashes
Warnings
For external use only
Do not use
- on children under 2 years of age. Consult a doctor.
- on mucous membranes
When using this product
- avoid contact with the eyes
- use in a well ventilated area
- flammable until dry
Stop use and ask a doctor if
- condition worsens
- symptoms persist for more than 7 days
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- clean affected area with mild soap and water, dry thoroughly
- brush on twice daily
- allow to dry for 1 minute before coming into contact with clothing
- reapply if peeling
- children from 2-12 years of age ask a doctor
Other Information
- store at 15º to 30º C (59º to 86ºF)
- Keep bottle tightly closed or product will evaporate
Inactive Ingredients
vitamin E, specially-formulated flexible collodion
Questions?
call 1-877-566-5935
Carton 30mL
Scarguard MD Physicians FormulaHydrocortisone Silicone LIQUID
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!