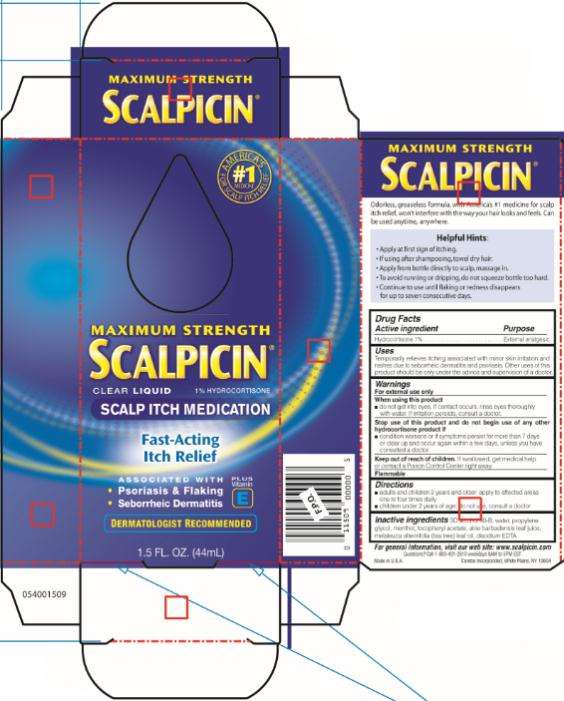
Scalpicin Scalp Itch Medication Maximum Strength
Scalpicin Scalp Itch Medication Maximum Strength
FULL PRESCRIBING INFORMATION
Maximum Strength Scalpicin ® Scalp Itch Medication
Hydrocortisone 1%
External analgesic
Temporarily relieves itching associated with minor skin irritation and rashes due to seborrheic dermatitis and psoriasis. Other uses of this product should be only under the advice and supervision of a doctor.
For external use only
- do not get into eyes. If contact occurs, rinse eyes thoroughly with water. If irritation persists, consult a doctor.
- condition worsens or if symptoms persist for more than 7 days or clear up and occur again with a few days, unless you have consulted a doctor
If swallowed, get medical help or contact a Poison Control Center right away.
Flammable
- adults and children 2 years and older: apply to affected areas one to four times daily
- children under 2 years of age: do not use, consult a doctor
SD alcohol 40-B, water, propylene glycol, menthol, tocopheryl acetate, aloe barbadensis leaf juice, melaleuca alternifolia (tea tree) leaf oil, disodium EDTA
visit our web site: www.scalpicin.com
Questions? Call 1-800-431-2610 weekdays 9AM to 5PM EST
Made in U.S.A. Combe Incorporated, White Plains, NY 10604
AMERICA’S
#1
MEDICINE
FOR SCALP ITCH RELIEF
MAXIMUM STRENGTH
SCALPICIN ®
CLEAR LIQUID 1 % HYDROCORTISONE
SCALP ITCH MEDICATION
Fast-Acting
Itch Relief
ASSOCIATED WITH
-
Psoriasis & Flaking
- Seborrheic Dermatitis
PLUS
Vitamin
E
DERMATOLOGIST RECOMMENDED
1.5 FL. OZ. (44 mL)
Scalpicin Scalp Itch Medication Maximum Strengthhydrocortisone LIQUID
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||