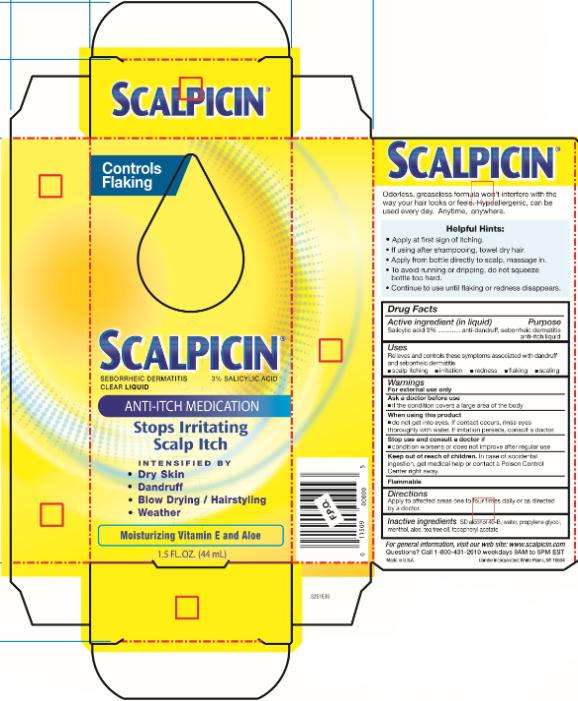
Scalpicin Anti Itch Medication
Scalpicin Anti Itch Medication
FULL PRESCRIBING INFORMATION
Scalpicin ® Anti-Itch Medication
Salicylic acid 3%
anti-dandruff, seborrheic dermatitis anti-itch liquid
Relieves and controls these symptoms associated with dandruff and seborrheic dermatitis
- scalp itching
- irritation
- redness
- flaking
- scaling
For external use only
- if the condition covers a large area of the body
- do not get into eyes. If contact occurs, rinse eyes thoroughly with water. If irritation persists, consult a doctor.
- condition worsens or does not improve after regular use
In case of accidental ingestion, get medical help or contact a Poison Control Center right away.
Flammable
Apply to affected areas one to four times daily or as directed by a doctor.
SD alcohol 40-B, water, propylene glycol, menthol, aloe, tea tree oil, tocopheryl acetate
visit our web site: www.scalpicin.com
Questions? Call 1-800-431-2610 weekdays 9AM to 5PM EST
Made in U.S.A. Combe Incorporated, White Plains, NY 10604
Controls
Flaking
Scalpicin ®
SEBORRHEIC
DERMATITIS
3% SALICYLIC ACID
CLEAR LIQUID
ANTI-ITCH MEDICATION
Stops Irritating
Scalp Itch
INTENSIFIED BY
-
Dry Skin
-
Dandruff
-
Blow Drying / Hairstyling
- Weather
Moisturizing Vitamin E and Aloe
1 . 5 FL. OZ. ( 44 mL )
VALUE
SIZE
Scalpicin ®
SEBORRHEIC
DERMATITIS
3% SALICYLIC ACID
CLEAR LIQUID
ANTI-ITCH MEDICATION
Stops Irritating
Scalp Itch
INTENSIFIED BY
-
Dry Skin
-
Dandruff
-
Blow Drying / Hairstyling
- Weather
Moisturizing Vitamin E and Aloe
2 . 5 FL. OZ. ( 74 mL )

Scalpicin Anti Itch Medicationsalicylic acid LIQUID
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||