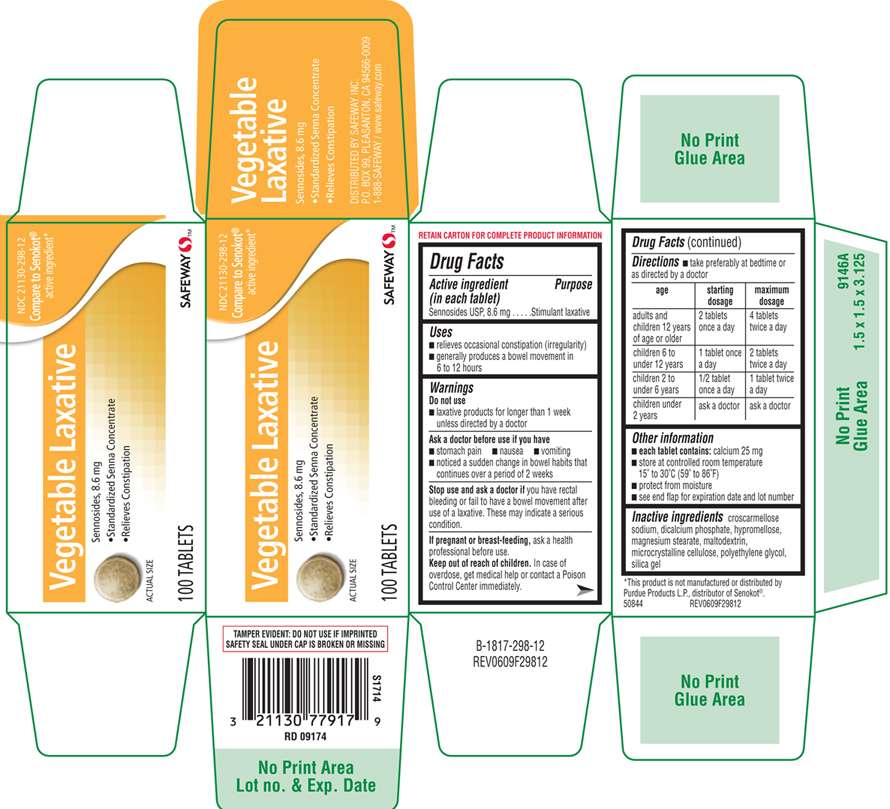
Safeway Sennosides
Safeway 44-298
FULL PRESCRIBING INFORMATION
Sennosides USP, 8.6 mg
Stimulant laxative
- relieves occasional constipation (irregularity)
- generally produces a bowel movement in 6 to 12 hours
- laxative products for longer than 1 week unless directed by a doctor
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- notice a sudden change in bowel habits that continues over a period of 2 weeks
you have rectal bleeding or fail to have a bowel movement after use of a laxative. These may indicate a serious condition.
ask a health professional before use.
In case of overdose, get medical help or contact a Poison Control Center immediately.
- take preferably at bedtime or as directed by a doctor
| age | starting dosage | maximum dosage |
| adults and children 12 years of age or older | 2 tablets once a day | 4 tablets twice a day |
| children 6 to under 12 years | 1 tablet once a day | 2 tablets twice a day |
| children 2 to under 6 years | 1/2 tablet once a day | 1 tablet twice a day |
| children under 2 years | ask a doctor | ask a doctor |
- each tablet contains: calcium 25 mg
- store at controlled room temperature 15° to 30°C (59° to 86°F)
- protect from moisture
- see end flap for expiration date and lot number
croscarmellose sodium, dicalcium phosphate, hypromellose, magnesium stearate, maltodextrin, microcrystalline cellulose, polyethylene glycol, silica gel
SAFEWAY™
Vegetable Laxative
Sennosides, 8.6 mg
- Standardized Senna Concentrate
- Relieves Constipation
ACTUAL SIZE
100 TABLETS
NDC 21130-298-12
Compare to Senokot
active ingredient
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFEY SEAL UNDER CAP IS BROKEN OR MISSING
50844 REV0609F29812
DISTRIBUTED BY SAFEWAY INC.
PO. BOX 99 PLEASENTON, CA 94566-0009
1-888-SAFEWAY / www.safeway.com
Safeway SennosidesSennosides TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||