Ropinirole Hydrochloride
Heritage Pharmaceuticals Inc.
Alembic Limited
Ropinirole Hydrochloride Tablets Rx only PRESCRIBING INFORMATION Patient Information Included
FULL PRESCRIBING INFORMATION: CONTENTS*
- ROPINIROLE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- ROPINIROLE HYDROCHLORIDE INDICATIONS AND USAGE
- ROPINIROLE HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- ROPINIROLE HYDROCHLORIDE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- ROPINIROLE HYDROCHLORIDE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- SPL Patient Package Insert
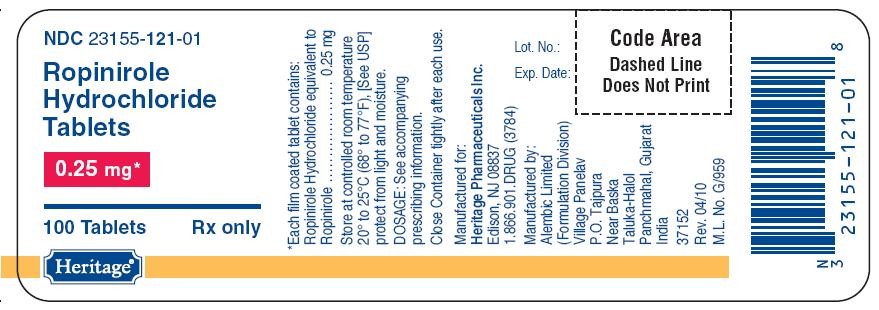
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
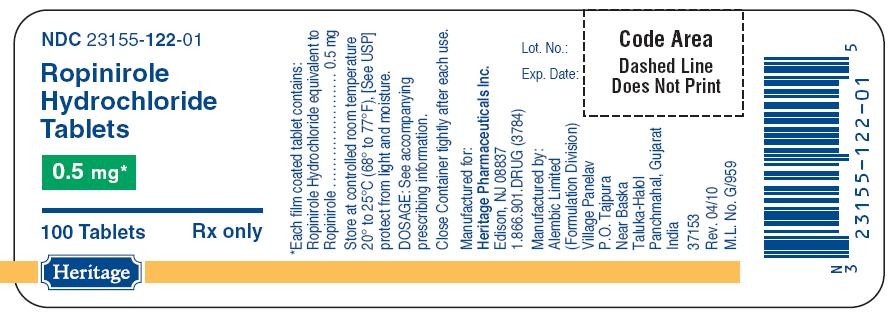
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
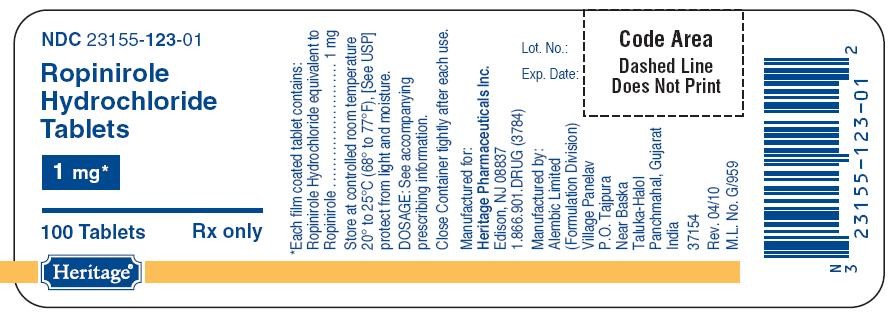
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
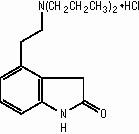
ROPINIROLE HYDROCHLORIDE DESCRIPTION
H16242
CLINICAL PHARMACOLOGY
Mechanism of Action23324
11212
Parkinson’s Disease
23
Restless Legs Syndrome (RLS)
Clinical Pharmacology Studies
2
Pharmacokinetics
Absorption, Distribution, Metabolism, and Elimination
maxmax
In vitro450
P450 Interaction
In vitro
Population Subgroups
Age
Gender
Race
Cigarette Smoking
max
Renal Impairment
Hepatic Impairment
Other Diseases
Clinical Trials
Parkinson's Disease
Studies in Patients With Early Parkinson’s Disease (Without L-dopa)
Study in Patients With Advanced Parkinson’s Disease (With L-dopa)
Restless Legs Syndrome (RLS)
INDICATIONS AND USAGE
Table 1. Mean Change in IRLS Score and Percent Responders on CGI-I
| Ropinirole Hydrochloride Tablets | Placebo | p-value | |
| Mean Change in IRLS score at Week 12 | |||
| US study | -13.5 | -9.8 | p<0.0001 |
| Multinational study (excluding US) | -11.0 | -8.0 | p=0.0036 |
| Multinational study (including US) | -11.2 | -8.7 | p=0.0197 |
| Percent responders on CGI-I at Week 12 | |||
| US study | 73.3% | 56.5% | p=0.0006 |
| Multinational study (excluding US) | 53.4% | 40.9% | p=0.0416 |
| Multinational study (including US) | 59.5% | 39.6% | p=0.0010 |
ROPINIROLE HYDROCHLORIDE INDICATIONS AND USAGE
Parkinson’s DiseaseThe effectiveness of Ropinirole Hydrochloride Tablets were demonstrated in randomized, controlled trials in patients with early Parkinson’s disease who were not receiving concomitant L-dopa therapy as well as in patients with advanced disease on concomitant L-dopa (see CLINICAL PHARMACOLOGY: Clinical Trials).
Restless Legs Syndrome
Key diagnostic criteria for RLS are: an urge to move the legs usually accompanied or caused by uncomfortable and unpleasant leg sensations; symptoms begin or worsen during periods of rest or inactivity such as lying or sitting; symptoms are partially or totally relieved by movement such as walking or stretching at least as long as the activity continues; and symptoms are worse or occur only in the evening or night. Difficulty falling asleep may frequently be associated with moderate-to-severe RLS.
ROPINIROLE HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
Falling Asleep During Activities of Daily LivingPatients treated with Ropinirole Hydrochloride Tablets have reported falling asleep while engaged in activities of daily living, including the operation of motor vehicles, which sometimes resulted in accidents. Although many of these patients reported somnolence while on Ropinirole Hydrochloride Tablets, some perceived that they had no warning signs such as excessive drowsiness, and believed that they were alert immediately prior to the event. Some of these events have been reported as late as 1 year after initiation of treatment.
In controlled clinical trials, somnolence was a common occurrence in patients receiving Ropinirole Hydrochloride Tablets and is more frequent in Parkinson's disease (up to 40% Ropinirole Hydrochloride Tablets, 6% placebo) than in Restless Legs Syndrome (12% Ropinirole Hydrochloride Tablets, 6% placebo). Many clinical experts believe that falling asleep while engaged in activities of daily living always occurs in a setting of preexisting somnolence, although patients may not give such a history. For this reason, prescribers should continually reassess patients for drowsiness or sleepiness, especially since some of the events occur well after the start of treatment. Prescribers should also be aware that patients may not acknowledge drowsiness or sleepiness until directly questioned about drowsiness or sleepiness during specific activities.
Before initiating treatment with Ropinirole Hydrochloride Tablets, patients should be advised of the potential to develop drowsiness and specifically asked about factors that may increase the risk with Ropinirole Hydrochloride Tablets such as concomitant sedating medications, the presence of sleep disorders (other than Restless Legs Syndrome), and concomitant medications that increase ropinirole plasma levels (e.g., ciprofloxacin - see PRECAUTIONS: Drug Interactions). If a patient develops significant daytime sleepiness or episodes of falling asleep during activities that require active participation (e.g., conversations, eating, etc.), Ropinirole Hydrochloride Tablets should ordinarily be discontinued. (See DOSAGE AND ADMINISTRATION for guidance in discontinuing Ropinirole Hydrochloride Tablets.) If a decision is made to continue Ropinirole Hydrochloride Tablets, patients should be advised to not drive and to avoid other potentially dangerous activities. There is insufficient information to establish that dose reduction will eliminate episodes of falling asleep while engaged in activities of daily living.
Syncope
Syncope, sometimes associated with bradycardia, was observed in association with ropinirole in both Parkinson’s disease patients and RLS patients. In the 2 double-blind, placebo-controlled studies of Ropinirole Hydrochloride Tablets in patients with Parkinson’s disease who were not being treated with L-dopa, 11.5% (18 of 157) of patients on Ropinirole Hydrochloride Tablets had syncope compared to 1.4% (2 of 147) of patients on placebo. Most of these cases occurred more than 4 weeks after initiation of therapy with Ropinirole Hydrochloride Tablets, and were usually associated with a recent increase in dose.
Of 208 patients being treated with both L-dopa and Ropinirole Hydrochloride Tablets in placebo-controlled advanced Parkinson’s disease trials, there were reports of syncope in 6 (2.9%) compared to 2 of 120 (1.7%) of placebo/L-dopa patients.
In patients with RLS, of 496 patients treated with Ropinirole Hydrochloride Tablets in 12-week placebo-controlled trials, there were reports of syncope in 5 (1.0%) compared with 1 of 500 (0.2%) patients treated with placebo.
Because the studies of Ropinirole Hydrochloride Tablets excluded patients with significant cardiovascular disease, it is not known to what extent the estimated incidence figures apply to either Parkinson’s disease or RLS patients in clinical practice. Therefore, patients with severe cardiovascular disease should be treated with caution.
Symptomatic Hypotension
Dopamine agonists, in clinical studies and clinical experience, appear to impair the systemic regulation of blood pressure, with resulting postural hypotension, especially during dose escalation. Parkinson’s disease patients, in addition, appear to have an impaired capacity to respond to a postural challenge. For these reasons, Parkinson’s patients being treated with dopaminergic agonists ordinarily (1) require careful monitoring for signs and symptoms of postural hypotension, especially during dose escalation, and (2) should be informed of this risk (see PRECAUTIONS: Information for Patients).
Although the clinical trials were not designed to systematically monitor blood pressure, there were individual reported cases of postural hypotension in early Parkinson’s disease (without L-dopa) in patients treated with Ropinirole Hydrochloride Tablets. Most of these cases occurred more than 4 weeks after initiation of therapy with Ropinirole Hydrochloride Tablets and were usually associated with a recent increase in dose.
In 12-week placebo-controlled trials of patients with RLS, the adverse event orthostatic hypotension was reported by 4 of 496 patients (0.8%) treated with Ropinirole Hydrochloride Tablets compared with 2 of 500 patients (0.4%) receiving placebo.
In two phase 2 studies in patients with RLS that used a forced-titration regimen and orthostatic challenges with intensive blood pressure monitoring, 14 of 55 patients (25%) receiving Ropinirole Hydrochloride Tablets experienced an adverse event of hypotension or postural hypotension. As described above, one additional patient was noted to have an episode of vasovagal syncope (although no blood pressure recording was documented). None of the 27 patients receiving placebo had a similar adverse event. In these studies, 11 of the 55 patients (20%) receiving Ropinirole Hydrochloride Tablets and 3 of the 26 patients (12%) who had post-dose blood pressure assessments following placebo, experienced an orthostatic blood pressure decrease of at least 40 mm Hg systolic and/or at least 20 mm Hg diastolic; not all of these changes were associated with clinical symptoms. Except for its forced nature these studies used a similar titration schedule as those in the phase 3 efficacy trials.
In phase 1 studies of Ropinirole Hydrochloride Tablets that included 110 healthy volunteers, 9 subjects had documented symptomatic postural hypotension. These episodes appeared mainly at doses above 0.8 mg and these doses are higher than the starting doses recommended for either Parkinson’s disease patients or RLS patients. In 8 of these 9 individuals, the hypotension was accompanied by bradycardia, but did not develop into syncope (see Syncope subsection). None of these events resulted in death or hospitalization.
Hallucinations
PRECAUTIONS
General
Dyskinesia
Renal Impairment
Hepatic Impairment
Events Reported With Dopaminergic Therapy
Withdrawal-Emergent Hyperpyrexia and Confusion
Fibrotic Complications
Melanoma
any
Augmentation and Rebound in RLS
Retinal Pathology
Albino Rats
2
Human
Binding to Melanin
Information for Patients
Impulse Control Symptoms Including Compulsive Behaviors:
see PRECAUTIONS: Pregnancy
Drug Interactions
P450 Interaction
In vitro
L-dopa
®max
Digoxin
Theophylline
Ciprofloxacin
max
Estrogens
Dopamine Antagonists
Carcinogenesis, Mutagenesis, Impairment of Fertility
222
in vitroin vitroin vivo
222
Pregnancy
Pregnancy Category C.2222
Nursing Mothers
Pediatric Use
ROPINIROLE HYDROCHLORIDE ADVERSE REACTIONS
Parkinson’s Disease
Early Parkinson’s Disease (Without L-dopa)
CLINICAL PHARMACOLOGY: Clinical Trials
Adverse Event Incidence in Controlled Clinical Studies
Table 2
Table 2. Treatment-Emergent Adverse Event * Incidence in Double-Blind, Placebo-Controlled Early Parkinson’s Disease (Without L-dopa) Trials (Events ≥2% of Patients Treated With Ropinirole Hydrochloride Tablets and Numerically More Frequent Than the Placebo Group)
| Adverse Experience | Ropinirole Hydrochloride Tablets (n = 157) (%) |
Placebo (n = 147) (%) |
| Autonomic nervous system | ||
| Flushing | 3 | 1 |
| Dry mouth | 5 | 3 |
| Increased sweating | 6 | 4 |
| Body as a whole | ||
| Asthenia | 6 | 1 |
| Chest pain | 4 | 2 |
| Dependent edema | 6 | 3 |
| Leg edema | 7 | 1 |
| Fatigue | 11 | 4 |
| Malaise | 3 | 1 |
| Pain | 8 | 4 |
| Cardiovascular general | ||
| Hypertension | 5 | 3 |
| Hypotension | 2 | 0 |
| Orthostatic symptoms | 6 | 5 |
| Syncope | 12 | 1 |
| Central/peripheral nervous system | ||
| Dizziness | 40 | 22 |
| Hyperkinesia | 2 | 1 |
| Hypesthesia | 4 | 2 |
| Vertigo | 2 | 0 |
| Gastrointestinal system | ||
| Abdominal pain | 6 | 3 |
| Anorexia | 4 | 1 |
| Dyspepsia | 10 | 5 |
| Flatulence | 3 | 1 |
| Nausea | 60 | 22 |
| Vomiting | 12 | 7 |
| Heart rate/rhythm | ||
| Extrasystoles | 2 | 1 |
| Atrial fibrillation | 2 | 0 |
| Palpitation | 3 | 2 |
| Tachycardia | 2 | 0 |
| Metabolic/nutritional | ||
| Increased alkaline phosphatase | 3 | 1 |
| Psychiatric | ||
| Amnesia | 3 | 1 |
| Impaired concentration | 2 | 0 |
| Confusion | 5 | 1 |
| Hallucination | 5 | 1 |
| Somnolence | 40 | 6 |
| Yawning | 3 | 0 |
| Reproductive male | ||
| Impotence | 3 | 1 |
| Resistance mechanism | ||
| Viral infection | 11 | 3 |
| Respiratory system | ||
| Bronchitis | 3 | 1 |
| Dyspnea | 3 | 0 |
| Pharyngitis | 6 | 4 |
| Rhinitis | 4 | 3 |
| Sinusitis | 4 | 3 |
| Urinary system | ||
| Urinary tract infection | 5 | 4 |
| Vascular extracardiac | ||
| Peripheral ischemia | 3 | 0 |
| Vision | ||
| Eye abnormality | 3 | 1 |
| Abnormal vision | 6 | 3 |
| Xerophthalmia | 2 | 0 |
Advanced Parkinson’s Disease (With L-dopa)
Adverse Event Incidence in Controlled Clinical Studies
Table 3
Table 3. Treatment-Emergent Adverse Event* Incidence in Double-Blind, Placebo-Controlled Advanced Parkinson’s Disease (With L-dopa) Trials (Events ≥2% of Patients Treated With Ropinirole Hydrochloride Tablets and Numerically More Frequent Than the Placebo Group)
| Adverse Experience |
Ropinirole Hydrochloride Tablets (n = 208) (%) |
Placebo (n = 120) (%) |
| Autonomic nervous system | ||
| Dry mouth | 5 | 1 |
| Increased sweating | 7 | 2 |
| Body as a whole | ||
| Increased drug level | 7 | 3 |
| Pain | 5 | 3 |
| Cardiovascular general | ||
| Hypotension | 2 | 1 |
| Syncope | 3 | 2 |
| Central/peripheral nervous system | ||
| Dizziness | 26 | 16 |
| Dyskinesia | 34 | 13 |
| Falls | 10 | 7 |
| Headache | 17 | 12 |
| Hypokinesia | 5 | 4 |
| Paresis | 3 | 0 |
| Paresthesia | 5 | 3 |
| Tremor | 6 | 3 |
| Gastrointestinal system | ||
| Abdominal pain | 9 | 8 |
| Constipation | 6 | 3 |
| Diarrhea | 5 | 3 |
| Dysphagia | 2 | 1 |
| Flatulence | 2 | 1 |
| Nausea | 30 | 18 |
| Increased saliva | 2 | 1 |
| Vomiting | 7 | 4 |
| Metabolic/nutritional | ||
| Weight decrease | 2 | 1 |
| Musculoskeletal system | ||
| Arthralgia | 7 | 5 |
| Arthritis | 3 | 1 |
| Psychiatric | ||
| Amnesia | 5 | 1 |
| Anxiety | 6 | 3 |
| Confusion | 9 | 2 |
| Abnormal dreaming | 3 | 2 |
| Hallucinations | 10 | 4 |
| Nervousness | 5 | 3 |
| Somnolence | 20 | 8 |
| Red blood cell | ||
| Anemia | 2 | 0 |
| Resistance mechanism | ||
| Upper respiratory tract infection | 9 | 8 |
| Respiratory system | ||
| Dyspnea | 3 | 2 |
| Urinary system | ||
| Pyuria | 2 | 1 |
| Urinary incontinence | 2 | 1 |
| Urinary tract infection | 6 | 3 |
| Vision | ||
| Diplopia | 2 | 1 |
Restless Legs Syndrome
Table 4DOSAGE AND ADMINISTRATION: General Dosing Considerations
Adverse Event Incidence in Controlled Clinical Studies
Table 4
Table 4. Treatment-Emergent Adverse Event Incidence in Double-Blind, Placebo-Controlled RLS Trials (Events ≥2% of Patients Treated With Ropinirole Hydrochloride Tablets and Numerically More Frequent Than the Placebo Group)
| Adverse Experience |
Ropinirole Hydrochloride Tablets (n = 496) (%) |
Placebo (n =500) (%) |
| Ear and labyrinth disorders | ||
| Vertigo | 2 | 1 |
| Gastrointestinal disorders | ||
| Nausea | 40 | 8 |
| Vomiting | 11 | 2 |
| Diarrhea | 5 | 3 |
| Dyspepsia | 4 | 3 |
| Dry mouth | 3 | 2 |
| Abdominal pain upper | 3 | 1 |
| General disorders and administration site conditions | ||
| Fatigue | 8 | 4 |
| Edema peripheral | 2 | 1 |
| Infections and infestations | ||
| Nasopharyngitis | 9 | 8 |
| Influenza | 3 | 2 |
| Musculoskeletal and connective tissue disorders | ||
| Arthralgia | 4 | 3 |
| Muscle cramps | 3 | 2 |
| Pain in extremity | 3 | 2 |
| Nervous system disorders | ||
| Somnolence | 12 | 6 |
| Dizziness | 11 | 5 |
| Paresthesia | 3 | 1 |
| Respiratory, thoracic, and mediastinal disorders | ||
| Cough | 3 | 2 |
| Nasal congestion | 2 | 1 |
| Skin and subcutaneous tissue disorders | ||
| Hyperhidrosis | 3 | 1 |
Other Adverse Events Observed During All Phase 2/3 Clinical Trials for Parkinson’s Disease
Body as a Whole
Infrequent: Rare:
Cardiovascular
Infrequent: Rare:
Central/Peripheral Nervous System
Frequent: Infrequent: Rare:
Endocrine
Infrequent: Rare:
Gastrointestinal
Infrequent: Rare:
Hematologic
Infrequent:
Metabolic/Nutritional
Frequent: Infrequent: Rare:
Musculoskeletal
Infrequent: Rare:
Neoplasm
Infrequent: Rare:
Psychiatric
Infrequent: Rare:
Genitourinary
Infrequent: Rare:
Resistance Mechanism
Infrequent:
Respiratory
Infrequent:
Skin/Appendage
Infrequent:
Special Senses
Infrequent: Rare:
Vascular Extracardiac
Infrequent: Rare
Falling Asleep During Activities of Daily Living
WARNING
Other Adverse Events Observed During Phase 2/3 Clinical Trials for RLS
Blood and Lymphatic System Disorders
Infrequent:
Cardiac Disorders
Frequent: Infrequent:
Congenital, Familial, and Genetic Disorders
Infrequent:
Ear and Labyrinth Disorders
Infrequent:
Endocrine Disorders
Infrequent:
Eye Disorders
Infrequent:
Gastrointestinal Disorders
Frequent: Infrequent:
General Disorders and Administration Site Conditions
Frequent: Infrequent:
Hepatobiliary Disorders
Infrequent:
Immune System Disorders
Infrequent:
Infections and Infestations
Frequent: Infrequent:
Injury, Poisoning, and Procedural Complications
Infrequent:
Investigations
Infrequent:
Metabolism and Nutrition Disorders
Infrequent:
Musculoskeletal and Connective Tissue Disorders
Frequent: Infrequent:
Neoplasms Benign, Malignant, and Unspecified
Infrequent:
Nervous System Disorders
Frequent: Infrequent:
Psychiatric Disorders
Frequent: Infrequent:
Renal and Urinary Disorders
Infrequent:
Reproductive System and Breast Disorders
Frequent: Infrequent:
Respiratory, Thoracic and Mediastinal Disorders
Frequent: Infrequent:
Skin and Subcutaneous Tissue Disorders
Frequent: Infrequent:
Vascular Disorders
Frequent: Infrequent:
Postmarketing Reports
Psychiatric Disorders:
DRUG ABUSE AND DEPENDENCE
CONTROLLED SUBSTANCE CLASS
Physical and Psychological Dependence
OVERDOSAGE
In the Parkinson's disease program, there have been patients who accidentally or intentionally took more than their prescribed dose of ropinirole. The largest overdose reported in the Parkinson's disease clinical trials was 435 mg taken over a 7-day period (62.1 mg/day). Of patients who received a dose greater than 24 mg/day, reported symptoms included adverse events commonly reported during dopaminergic therapy (nausea, dizziness), as well as visual hallucinations, hyperhidrosis, claustrophobia, chorea, palpitations, asthenia, and nightmares. Additional symptoms reported for doses of 24 mg or less or for overdoses of unknown amount included vomiting, increased coughing, fatigue, syncope, vasovagal syncope, dyskinesia, agitation, chest pain, orthostatic hypotension, somnolence, and confusional state.
Overdose Management
ROPINIROLE HYDROCHLORIDE DOSAGE AND ADMINISTRATION
General Dosing Considerations for Parkinson's Disease and RLS
Geriatric Use
CLINICAL PHARMACOLOGY
Renal Impairment
CLINICAL PHARMACOLOGY
Hepatic Impairment
Dosing for Parkinson’s Disease
Table 5
Table 5. Ascending-Dose Schedule of Ropinirole Hydrochloride Tablets for Parkinson’s Disease
| Week |
Dosage |
Total Daily Dose |
| 1 |
0.25 mg 3 times daily | 0.75 mg |
| 2 |
0.5 mg 3 times daily | 1.5 mg |
| 3 |
0.75 mg 3 times daily | 2.25 mg |
| 4 |
1 mg 3 times daily | 3 mg |
Dosing for Restless Legs Syndrome
Table 6
Table 6. Dose Titration Schedule for RLS
| Day/Week |
Dosage to be taken once daily, 1 to 3 hours before bedtime |
| Days 1 and 2 |
0.25 mg |
| Days 3-7 |
0.5 mg |
| Week 2 |
1 mg |
| Week 3 |
1.5 mg |
| Week 4 |
2 mg |
| Week 5 |
2.5 mg |
| Week 6 |
3 mg |
| Week 7 |
4 mg |
HOW SUPPLIED
Tablets
STORAGE: Protect from light and moisture. Close container tightly after each use.Store at controlled room temperature 20°-25°C (68°-77°F) [see USP].

Heritage Pharmaceuticals Inc.
SPL Patient Package Insert
PATIENT INFORMATION
ROPINIROLE HYDROCHLORIDE TABLETS
If you have Restless Legs Syndrome (RLS, also known as Ekbom Syndrome), read this side
Read this information completely before you start taking Ropinirole Hydrochloride Tablets.
What is Ropinirole Hydrochloride Tablets?
What is the most important information I should know about Ropinirole Hydrochloride Tablets?
- Patients with RLS should take Ropinirole Hydrochloride Tablets differently than patients with Parkinson's disease (see How should I take Ropinirole Hydrochloride Tablets for RLS?for the recommended dosing for RLS).A lower dose of Ropinirole Hydrochloride Tablets is generally needed for patients with RLS, and is taken once daily before bedtime.
- There are known side effects of Ropinirole Hydrochloride Tablets. If you fall asleep or feel very sleepy while doing normal activities such as driving, faint, feel dizzy, nauseated, or sweaty when you stand up from sitting or lying down, you should talk with your doctor (see What are the possible side effects of Ropinirole Hydrochloride Tablets?).
- Before starting Ropinirole Hydrochloride Tablets, be sure to tell your doctor if you are taking any medicines that make you drowsy.
Who should not take Ropinirole Hydrochloride Tablets?
What should I tell my doctor?
- you are pregnant or plan to become pregnant.
- you are breast-feeding.
- you have daytime sleepiness from a sleep disorder other than RLS or have unexpected sleepiness or periods of sleep while taking Ropinirole Hydrochloride Tablets.
- you are taking any other prescription or over-the-counter medicines. Some of these medicines may increase your chances of getting side effects while taking Ropinirole Hydrochloride Tablets.
- you start or stop taking other medicines while you are taking Ropinirole Hydrochloride Tablets. This may increase your chances of getting side effects.
- you start or stop smoking while you are taking Ropinirole Hydrochloride Tablets. Smoking may decrease the treatment effect of Ropinirole Hydrochloride Tablets.
- you feel dizzy, nauseated, sweaty, or faint when you stand up from sitting or lying down.
- you drink alcoholic beverages. This may increase your chances of becoming drowsy or sleepy while taking Ropinirole Hydrochloride Tablets.
How should I take Ropinirole Hydrochloride Tablets for RLS?
- Be sure to take Ropinirole Hydrochloride Tablets exactly as directed by your doctor or healthcare provider.
- The usual way to take Ropinirole Hydrochloride Tablets is once in the evening, 1 to 3 hours before bedtime.
- Your doctor will start you on a low dose of Ropinirole Hydrochloride Tablets. Your doctor may change the dose until you are taking the amount of medicine that is right for you to control your symptoms.
- If you miss your dose, do not double your next dose . Take only your usual dose 1 to 3 hours before your next bedtime.
- Contact your doctor, if you stop taking Ropinirole Hydrochloride Tablets for any reason. Do not restart without consulting your doctor.
- You can take Ropinirole Hydrochloride Tablets with or without food. Taking Ropinirole Hydrochloride Tablets with food may decrease the chances of feeling nauseated.
What are the possible side effects of Ropinirole Hydrochloride Tablets?
- Most people who take Ropinirole Hydrochloride Tablets tolerate it well. The most commonly reported side effects in people taking Ropinirole Hydrochloride Tablets for RLS are nausea, vomiting, dizziness, and drowsiness or sleepiness. You should be careful until you know if Ropinirole Hydrochloride Tablets affects your ability to remain alert while doing normal daily activities, and you should watch for the development of significant daytime sleepiness or episodes of falling asleep. It is possible that you could fall asleep while doing normal activities such as driving a car, doing physical tasks, or using hazardous machinery while taking Ropinirole Hydrochloride Tablets. Your chances of falling asleep while doing normal activities while taking Ropinirole Hydrochloride Tablets are greater if you are taking other medicines that cause drowsiness.
- When you start taking Ropinirole Hydrochloride Tablets or when you increase your dose, you may feel dizzy, nauseated, sweaty or faint, when first standing up from sitting or lying down. Therefore, do not stand up quickly after sitting or lying down, particularly if you have been sitting or lying down for a long period of time. Take a minute sitting on the edge of the bed or chair before you get up.
- Some patients taking ropinirole have shown urges to behave in a way unusual for them. Examples of this are an unusual urge to gamble or increased sexual urges and/or behaviors. If you or your family notices that you are developing any unusual behaviors, talk to your doctor.
- Hallucinations (unreal sounds, visions, or sensations) have been reported in patients taking Ropinirole Hydrochloride Tablets. These were uncommon in patients taking Ropinirole Hydrochloride Tablets for RLS. The risk is greater in patients with Parkinson's disease who are elderly, taking Ropinirole Hydrochloride Tablets with L-dopa, or taking higher doses of Ropinirole Hydrochloride than recommended for RLS.
Other Information about Ropinirole Hydrochloride Tablets
- Studies of people with Parkinson’s disease show that they may be at an increased risk of developing melanoma, a form of skin cancer, when compared to people without Parkinson’s disease. It is not known if this problem is associated with Parkinson’s disease or the medicines used to treat Parkinson’s disease. Ropinirole Hydrochloride Tablets is one of the medicines used to treat Parkinson’s disease, therefore, patients being treated with Ropinirole Hydrochloride Tablets should have periodic skin examinations.
- Take Ropinirole Hydrochloride Tablets exactly as your doctor prescribes it.
- Do not share Ropinirole Hydrochloride Tablets with other people, even if they have the same symptoms you have.
- Keep Ropinirole Hydrochloride Tablets out of the reach of children.
- Store Ropinirole Hydrochloride Tablets at room temperature out of direct sunlight.
- Keep Ropinirole Hydrochloride Tablets in a tightly closed container.

PATIENT INFORMATION
ROPINIROLE HYDROCHLORIDE TABLETS
If you have Parkinson's desease, read this side
Read this information completely before you start taking Ropinirole Hydrochloride Tablets
What is Ropinirole Hydrochloride Tablets?
What is the most important information I should know about Ropinirole Hydrochloride Tablets?
- Patients with Parkinson's disease should take Ropinirole Hydrochloride Tablets differently than patients with Restless Legs Syndrome (see How should I take Ropinirole Hydrochloride Tablets for Parkinson's disease?). For Parkinson's disease, a higher dose of Ropinirole Hydrochloride Tablets is generally needed, and is taken more frequently throughout the day.
- There are known side effects of Ropinirole Hydrochloride Tablets (see What are the possible side effects of Ropinirole Hydrochloride Tablets?).
- If you fall asleep or feel very sleepy while doing normal activities such as driving, faint, feel dizzy, nauseated, or sweaty when you stand up from sitting or lying down, you should talk with your doctor.
- Hallucinations (unreal visions, sounds, or sensations) have been reported in patients taking Ropinirole Hydrochloride Tablets. The risk is greater in patients with Parkinson's disease who are elderly, taking Ropinirole Hydrochloride Tablets with L-dopa or taking higher doses of Ropinirole Hydrochloride Tablets. If these occur, you should discuss them with your doctor.
- Ropinirole Hydrochloride Tablets may make some of the side effects of L-dopa worse. Ropinirole Hydrochloride Tablets may cause uncontrolled sudden movements or make such movements you already have worse or more frequent. You should notify your doctor in such a case as dosage adjustments to your anti-Parkinson’s medications may be necessary.
- Before starting Ropinirole Hydrochloride Tablets, be sure to tell your doctor if you are taking any medicines that make you drowsy.
Who should not take Ropinirole Hydrochloride Tablets?
What should I tell my doctor?
- you are pregnant or plan to become pregnant.
- you are breast-feeding.
- you have daytime sleepiness from a sleep disorder or have unexpected sleepiness or periods of sleep while taking Ropinirole Hydrochloride Tablets.
- you are taking any other prescription or over-the-counter medicines. Some of these medicines may increase your chances of getting side effects while taking Ropinirole Hydrochloride Tablets.
- you start or stop taking other medicines while you are taking Ropinirole Hydrochloride Tablets. This may increase your chances of getting side effects.
- you start or stop smoking while you are taking Ropinirole Hydrochloride Tablets. Smoking may decrease the treatment effect of Ropinirole Hydrochloride Tablets.
- you feel dizzy, nauseated, sweaty, or faint when you first stand up from sitting or lying down.
- you drink alcoholic beverages. This may increase your chances of becoming drowsy or sleepy while taking Ropinirole Hydrochloride Tablets.
How should I take Ropinirole Hydrochloride Tablets for Parkinson's disease?
- Be sure to take your Ropinirole Hydrochloride Tablets exactly as directed by your doctor or healthcare provider.
- Three times a day is the usual way to take Ropinirole Hydrochloride Tablets for Parkinson's disease.
- Your doctor will start you on a low dose of Ropinirole Hydrochloride Tablets. Your doctor will change the dose until you are taking the right amount of medicine to control your symptoms. It may take several weeks before you reach a dose that controls your symptoms.
- If you miss a dose, do not double your next dose.
- Contact your doctor, if you stop taking Ropinirole Hydrochloride Tablets for any reason. Do not restart without consulting your doctor.
- Your doctor may prescribe Ropinirole Hydrochloride Tablets alone or add Ropinirole Hydrochloride Tablets to medicine that you are already taking for Parkinson's disease.
- You can take Ropinirole Hydrochloride Tablets with or without food. Taking Ropinirole Hydrochloride Tablets with food may decrease the chances of feeling nauseated.
What are the possible side effects of Ropinirole Hydrochloride Tablets?
- Most people who take Ropinirole Hydrochloride Tablets tolerate it well. The most commonly reported side effects in people taking Ropinirole Hydrochloride Tablets are nausea, headache, dizziness, drowsiness or sleepiness.
- You should be careful until you know if Ropinirole Hydrochloride Tablets affects your ability to remain alert while doing normal daily activities, and you should watch for the development of significant daytime sleepiness or episodes of falling asleep. It is possible that you could fall asleep while doing normal activities such as driving a car, doing physical tasks, or using hazardous machinery while taking Ropinirole Hydrochloride Tablets. Your chances of falling asleep while doing normal activities while taking Ropinirole Hydrochloride Tablets are greater if you are taking other medicines that cause drowsiness.
- When you start taking Ropinirole Hydrochloride Tablets or when you increase your dose, you may feel dizzy, nauseated, sweaty or faint, when first standing up from sitting or lying down. Therefore, do not stand up quickly after sitting or lying down, particularly if you have been sitting or lying down for a long period of time. Take a minute sitting on the edge of the bed or chair before you get up.
- Some patients taking ropinirole have shown urges to behave in a way unusual for them. Examples of this are an unusual urge to gamble or increased sexual urges and/or behaviors. If you or your family notices that you are developing any unusual behaviors, talk to your doctor.
- Hallucinations (unreal visions, sounds, or sensations) have been reported in patients taking Ropinirole Hydrochloride Tablets. The risk is greater in patients with Parkinson's disease who are elderly, taking Ropinirole Hydrochloride Tablets with L-dopa, or taking higher amounts of Ropinirole Hydrochloride Tablets.
- If you are taking L-dopa for Parkinson's disease, Ropinirole Hydrochloride Tablets may make some of the side effects of L-dopa worse. Ropinirole Hydrochloride Tablets may cause uncontrolled sudden movements or make such movements you already have worse or more frequent.
Other Information about Ropinirole Hydrochloride Tablets
- Studies of people with Parkinson’s disease show that they may be at an increased risk of developing melanoma, a form of skin cancer, when compared to people without Parkinson’s disease. It is not known if this problem is associated with Parkinson’s disease or the medicines used to treat Parkinson’s disease. Therefore, patients being treated with Ropinirole Hydrochloride Tablets should have periodic skin examinations.
- Take Ropinirole Hydrochloride Tablets exactly as your doctor prescribes it.
- Do not share Ropinirole Hydrochloride Tablets with other people, even if they have the same symptoms you have.
- Keep Ropinirole Hydrochloride Tablets out of the reach of children.
- Store Ropinirole Hydrochloride Tablets at room temperature out of direct sunlight.
- Keep Ropinirole Hydrochloride Tablets in a tightly closed container.

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
121
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
122
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
123
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
124

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
125
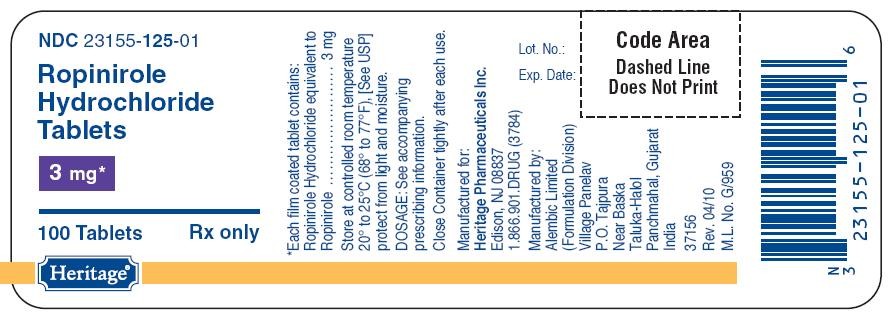
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
126

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
127
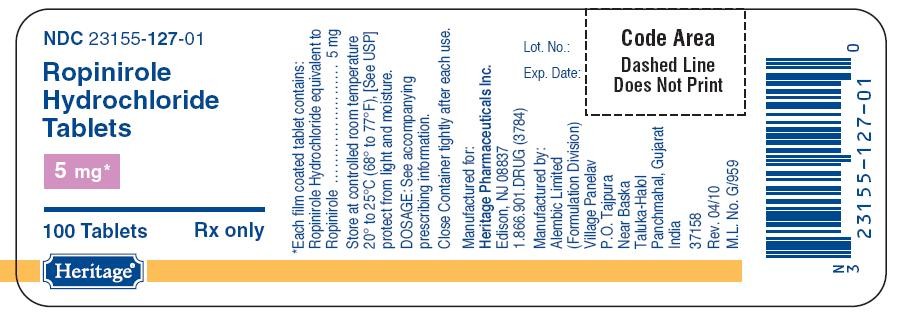
Ropinirole HydrochlorideRopinirole Hydrochloride TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Ropinirole HydrochlorideRopinirole Hydrochloride TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Ropinirole HydrochlorideRopinirole Hydrochloride TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Ropinirole HydrochlorideRopinirole Hydrochloride TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Ropinirole HydrochlorideRopinirole Hydrochloride TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Ropinirole HydrochlorideRopinirole Hydrochloride TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Ropinirole HydrochlorideRopinirole Hydrochloride TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||