Rodan and Fields
Cosmetic Enterprises Ltd.
Rodan & Fields, LLC.
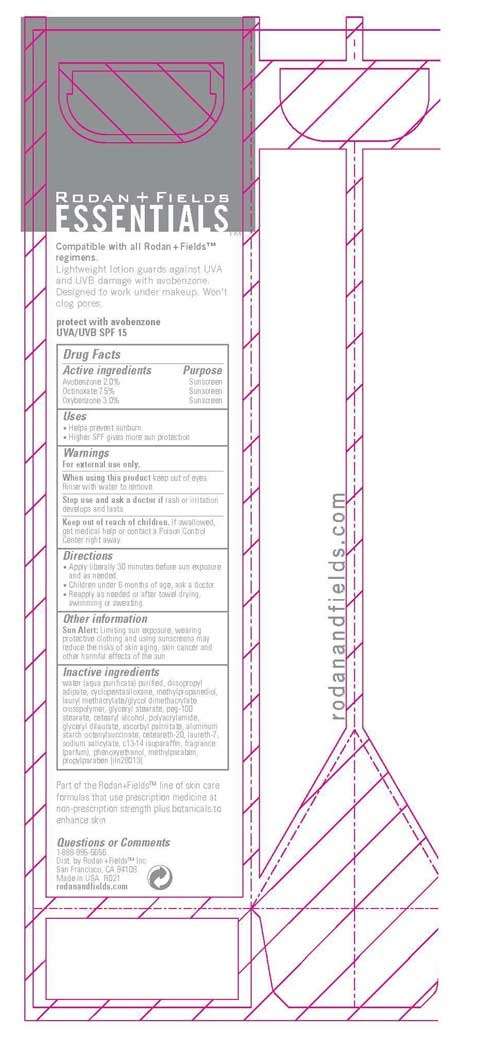
Essentials UVA/UVB SPF 15
FULL PRESCRIBING INFORMATION
Active ingredient
Active Ingredients Purpose
Avobenzone 2.0% Sunscreen
Octinoxate 7.5% Sunscreen
Oxybenzone 3.0 % Sunscreen
Warnings
For external use only.
When using this product keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash or irritation develops and lasts
Keep out of of children. If swallowed, get medical help or contact a Poison Control Center right away.
water (aqua purificata) purified, diisopropyl adipate, cyclopentasiloxane, methylpropanediol, lauryl methacrylate/glycol dimethacrylate crosspolymer, glyceryl stearate, peg-100 stearate, cetearyl alcohol, polyacrylamide, glyceryl dilaurate, ascorbyl palmitate, aluminum starch octenylsuccinate, ceteareth-20, laureth-7, sodium salicylate, c13-14 isoparaffin, fragrance (parfum), phenoxyethanol, methylparaben, propylparaben
Questions or Comments
1-888-895-5656
Sun Alert: Limiting sun exposure, wearing protective clothing, and using sunscreens may reduce the risks of skin aging, skin cancer and other harmful effects of the sun.
Essential UVA/UVB SPF 15
1.7 fl.oz./50 ml

Rodan and FieldsAvobenzone, Octinoxate, Oxybenzone CREAM
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||