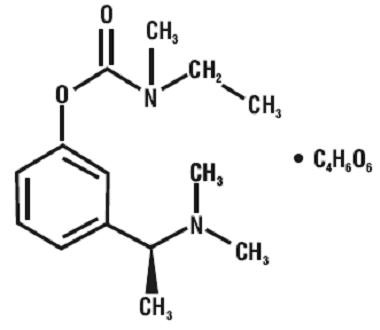
Rivastigmine Tartrate
Rivastigmine Tartrate Capsules
FULL PRESCRIBING INFORMATION: CONTENTS*
- RIVASTIGMINE TARTRATE DESCRIPTION
- CLINICAL PHARMACOLOGY
- RIVASTIGMINE TARTRATE INDICATIONS AND USAGE
- RIVASTIGMINE TARTRATE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- RIVASTIGMINE TARTRATE ADVERSE REACTIONS
- OVERDOSAGE
- RIVASTIGMINE TARTRATE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL-Bottle Label-6 mg
FULL PRESCRIBING INFORMATION
RIVASTIGMINE TARTRATE DESCRIPTION
142222·466
CLINICAL PHARMACOLOGY
Mechanism of Action
In vitroin vivo
Clinical Trial Data
Dementia of the Alzheimer’s type
Study Outcome Measures:
U.S. 26-Week Study
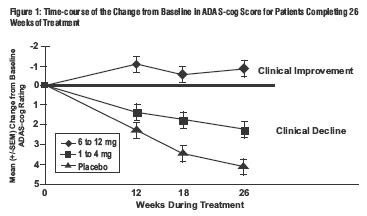
Effects on the ADAS-cog:

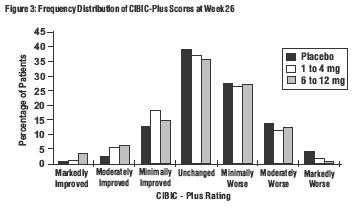
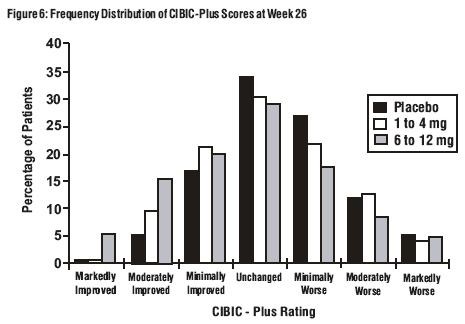
Effects on the CIBIC-Plus:
Global 26-Week Study
Effects on the ADAS-cog:
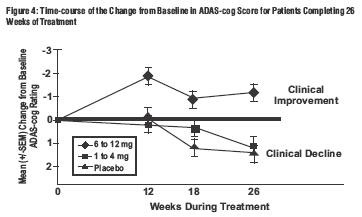
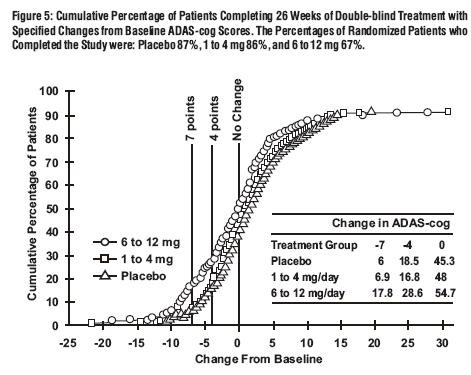
Effects on the CIBIC-Plus:
U.S. Fixed Dose Study
Dementia Associated with Parkinson’s disease (PDD)
International Twenty-Four-Week Study
Study Outcome Measures:
Study Results:
Effects on the ADAS-cog:

Effects on the ADCS-CGIC:
 Age, Gender and Race:
Age, Gender and Race: Pharmacokinetics
Absorption: maxmax
Distribution: 1-12hr
Metabolism: in vitro Drug-Drug Interactions
Elimination: 14
Special Populations
Hepatic Disease:
Renal Disease:
Age:
Gender and Race:
Nicotine Use:
Drug-Drug Interactions
Effect of Rivastigmine Tartrate Capsules on the Metabolism of Other Drugs: in vitro
Effect of Other Drugs on the Metabolism of Rivastigmine Tartrate Capsules:
RIVASTIGMINE TARTRATE INDICATIONS AND USAGE
CLINICAL PHARMACOLOGY, Clinical Trial Data
RIVASTIGMINE TARTRATE CONTRAINDICATIONS
DESCRIPTION
WARNINGS
Gastrointestinal Side Effects
Rivastigmine Tartrate Capsules use is associated with significant gastrointestinal adverse reactions, including nausea and vomiting, anorexia, and weight loss. For this reason, patients should always be started at a dose of 1.5 mg BID and titrated to their maintenance dose. If treatment is interrupted for longer than several days, treatment should be reinitiated with the lowest daily dose (see DOSAGE AND ADMINISTRATION) to reduce the possibility of severe vomiting and its potentially serious sequelae (e.g., there has been one postmarketing report of severe vomiting with esophageal rupture following inappropriate reinitiation of treatment with a 4.5 mg dose after 8 weeks of treatment interruption.)
Nausea and Vomiting: In the controlled clinical trials, 47% of the patients treated with Rivastigmine Tartrate Capsules dose in the therapeutic range of 6 to 12 mg/day (n=1189) developed nausea (compared with 12% in placebo). A total of 31% of Rivastigmine Tartrate Capsules -treated patients developed at least one episode of vomiting (compared with 6% for placebo). The rate of vomiting was higher during the titration phase (24% vs. 3% for placebo) than in the maintenance phase (14% vs. 3% for placebo). The rates were higher in women than men. Five percent of patients discontinued for vomiting, compared to less than 1% for patients on placebo. Vomiting was severe in 2% of Rivastigmine Tartrate Capsules -treated patients and was rated as mild or moderate each in 14% of patients. The rate of nausea was higher during the titration phase (43% vs. 9% for placebo) than in the maintenance phase (17% vs. 4% for placebo).
Weight Loss: In the controlled trials, approximately 26% of women on high doses of Rivastigmine Tartrate Capsules (greater than 9 mg/day) had weight loss equal to or greater than 7% of their baseline weight compared to 6% in the placebo-treated patients. About 18% of the males in the high dose group experienced a similar degree of weight loss compared to 4% in placebo-treated patients. It is not clear how much of the weight loss was associated with anorexia, nausea, vomiting, and the diarrhea associated with the drug.
Anorexia:
Peptic Ulcers/Gastrointestinal Bleeding:
Anesthesia
Cardiovascular Conditions
Genitourinary
Neurological Conditions
Seizures:
Pulmonary Conditions
PRECAUTIONS
Information for Patients and Caregivers
Drug-Drug Interactions
Effect of Rivastigmine Tartrate Capsules on the Metabolism of Other Drugs: in vitro
Effect of Other Drugs on the Metabolism of Rivastigmine Tartrate Capsules:
Use with Anticholinergics:
Use with Cholinomimetics and Other Cholinesterase Inhibitors:
Carcinogenesis, Mutagenesis, Impairment of Fertility
2
in vitro in vitro in vivo
2
Pregnancy
Pregnancy Category B: 2 22
Nursing Mothers
Pediatric Use
RIVASTIGMINE TARTRATE ADVERSE REACTIONS
Dementia of the Alzheimer's type
Adverse Events Leading to Discontinuation
| Study Phase | Titration | Maintenance | Overall | |||
|---|---|---|---|---|---|---|
|
Event/% Discontinuing
|
Placebo (n=868) |
Rivastigmine Tartrate Capsules ≥6 to 12 mg/day (n=1189) |
Placebo (n=788) |
Rivastigmine Tartrate Capsules ≥6 to 12 mg/day (n=987) |
Placebo (n=868) |
Rivastigmine Tartrate Capsules ≥6 to 12 mg/day (n=1189) |
| Nausea |
<1 |
8 |
<1 |
1 |
1 |
8 |
| Vomiting |
<1 |
4 |
<1 |
1 |
<1 |
5 |
| Anorexia |
0 |
2 |
<1 |
1 |
<1 |
3 |
| Dizziness |
<1 |
2 |
<1 |
1 |
<1 |
2 |
Gastrointestinal Adverse Reactions
WARNINGS
Adverse Events Reported in Controlled Trials
| Body System/Adverse Event | Placebo (n=868) |
Rivastigmine Tartrate Capsules (6 to 12 mg/day) (n=1189) |
|---|---|---|
| Percent of Patients with any Adverse Event | 79 |
92 |
|
Autonomic Nervous System
Sweating increased Syncope |
1 2 |
4 3 |
|
Body as a Whole
Accidental Trauma Fatigue Asthenia Malaise Influenza-like Symptoms Weight Decrease |
9 5 2 2 2 <1 |
10 9 6 5 3 3 |
|
Cardiovascular Disorders, General
Hypertension |
2 |
3 |
|
Central and Peripheral Nervous System
Dizziness Headache Somnolence Tremor |
11 12 3 1 |
21 17 5 4 |
|
Gastrointestinal System
Nausea Vomiting Diarrhea Anorexia Abdominal Pain Dyspepsia Constipation Flatulence Eructation |
12 6 11 3 6 4 4 2 1 |
47 31 19 17 13 9 5 4 2 |
|
Psychiatric Disorders
Insomnia Confusion Depression Anxiety Hallucination Aggressive Reaction |
7 7 4 3 3 2 |
9 8 6 5 4 3 |
|
Resistance Mechanism Disorders
Urinary Tract Infection |
6 |
7 |
|
Respiratory System
Rhinitis |
3 |
4 |
Dementia Associated with Parkinson's disease
Adverse Events leading to discontinuation
Most Frequent Adverse Clinical Events Seen in Association with the Use of Rivastigmine Tartrate Capsules
Adverse Events Reported in Controlled Trials
|
Body System/Adverse Event
|
Placebo
|
Rivastigmine Tartrate Capsules
|
|
(n=179)
|
(3
to
12 mg/day)
(n=362) |
|
|
Percent of Patients with any Adverse Event
|
71 |
84 |
|
Gastrointestinal disorders
|
|
|
| Nausea |
11 |
29 |
| Vomiting |
2 |
17 |
| Diarrhea |
4 |
7 |
| Upper abdominal pain |
1 |
4 |
|
General Disorders and administrative site conditions
|
|
|
| Fatigue |
3 |
4 |
| Asthenia |
1 |
2 |
|
Metabolism and nutritional disorders
|
|
|
| Anorexia |
3 |
6 |
| Dehydration |
1 |
2 |
|
Nervous system Disorders
|
|
|
| Tremor |
4 |
10 |
| Dizziness |
1 |
6 |
| Headache |
3 |
4 |
| Somnolence |
3 |
4 |
| Parkinson’s disease (worsening) |
1 |
3 |
| Parkinsonism |
1 |
2 |
|
Psychiatric Disorders
|
|
|
| Anxiety |
1 |
4 |
| Insomnia |
2 |
3 |
Other Adverse Events Observed During Clinical Trials
Dementia of the Alzheimer's Type
Autonomic Nervous System: Infrequent:
Body as a Whole: Frequent: Infrequent
Cardiovascular System: Frequent:
Central and Peripheral Nervous System: Frequent: Infrequent:
Endocrine System: Infrequent:
Gastrointestinal System: Frequent: Infrequent:
Hearing and Vestibular DisordersFrequent:
Heart Rate and Rhythm Disorders: Frequent: Infrequent:
Liver and Biliary System Disorders: Infrequent:
Metabolic and Nutritional Disorders: Frequent: Infrequent:
Musculoskeletal Disorders: Frequent: Infrequent:
Myo-, Endo-, Pericardial and Valve Disorders: Frequent:
Platelet, Bleeding, and Clotting Disorders: Frequent: Infrequent:
Psychiatric Disorders: Frequent: Infrequent:
Red Blood Cell Disorders: Frequent: Infrequent:
Reproductive Disorders (Female & Male): Infrequent:
Resistance Mechanism Disorders: Infrequent:
Respiratory System: Infrequent:
Skin and Appendages: Frequent: Infrequent:
Special Senses: Infrequent:
Urinary System Disorders: Frequent: Infrequent:
Vascular (extracardiac) Disorders: Infrequent:
Vision Disorders: Frequent: Infrequent:
White Cell and Resistance Disorders: Infrequent:
Dementia Associated with Parkinson's Disease
Cardiovascular System: Frequent:Infrequent:
Central and Peripheral Nervous System: Frequent:Infrequent:
Endocrine System: Infrequent:
Gastrointestinal System: Frequent: Infrequent:
Hearing and Vestibular Disorders: Frequent: Infrequent:
Heart Rate and Rhythm Disorders: Infrequent:
Liver and Biliary System Disorders: Infrequent:
Musculoskeletal Disorders: Frequent: Infrequent:
Psychiatric Disorders: Frequent: Infrequent:
Reproductive Disorders (Female & Male): Infrequent:
Respiratory System: Frequent: Infrequent:
Urinary System Disorders: Infrequent:
Vascular (extracardiac) Disorders: Infrequent:
Vision Disorders: Infrequent:
Post-Introduction Reports
Skin and Appendages:
OVERDOSAGE
Because strategies for the management of overdose are continually evolving, it is advisable to contact a Poison Control Center to determine the latest recommendations for the management of an overdose of any drug.
As Rivastigmine Tartrate Capsule has a short plasma half-life of about one hour and a moderate duration of acetylcholinesterase inhibition of 8 to 10 hours, it is recommended that in cases of asymptomatic overdoses, no further dose of Rivastigmine Tartrate Capsules should be administered for the next 24 hours.
As in any case of overdose, general supportive measures should be utilized. Overdosage with cholinesterase inhibitors can result in cholinergic crisis characterized by severe nausea, vomiting, salivation, sweating, bradycardia, hypotension, respiratory depression, collapse and convulsions. Increasing muscle weakness is a possibility and may result in death if respiratory muscles are involved. Atypical responses in blood pressure and heart rate have been reported with other drugs that increase cholinergic activity when co-administered with quaternary anticholinergics such as glycopyrrolate. Due to the short half-life of Rivastigmine Tartrate Capsules, dialysis (hemodialysis, peritoneal dialysis, or hemofiltration) would not be clinically indicated in the event of an overdose.
In overdoses accompanied by severe nausea and vomiting, the use of antiemetics should be considered. In a documented case of a 46 mg overdose with Rivastigmine Tartrate Capsules, the patient experienced vomiting, incontinence, hypertension, psychomotor retardation, and loss of consciousness. The patient fully recovered within 24 hours and conservative management was all that was required for treatment.
RIVASTIGMINE TARTRATE DOSAGE AND ADMINISTRATION
Dementia of the Alzheimer's type
WARNINGS
Dementia associated with Parkinson's Disease
HOW SUPPLIED
| Bottles of 60's with Child Resistant Cap |
NDC 54868-6145-0 |
Caraco Pharmaceutical Laboratories, Ltd.
Sun Pharmaceutical Industries Ltd.
Relabeling of "Additional Barcode Label" by:
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL-Bottle Label-6 mg
Rivastigmine Tartrate Capsules
equivalent to 6 mg base
Rx only
60 CAPSULES

Rivastigmine Tartraterivastigmine tartrate CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||