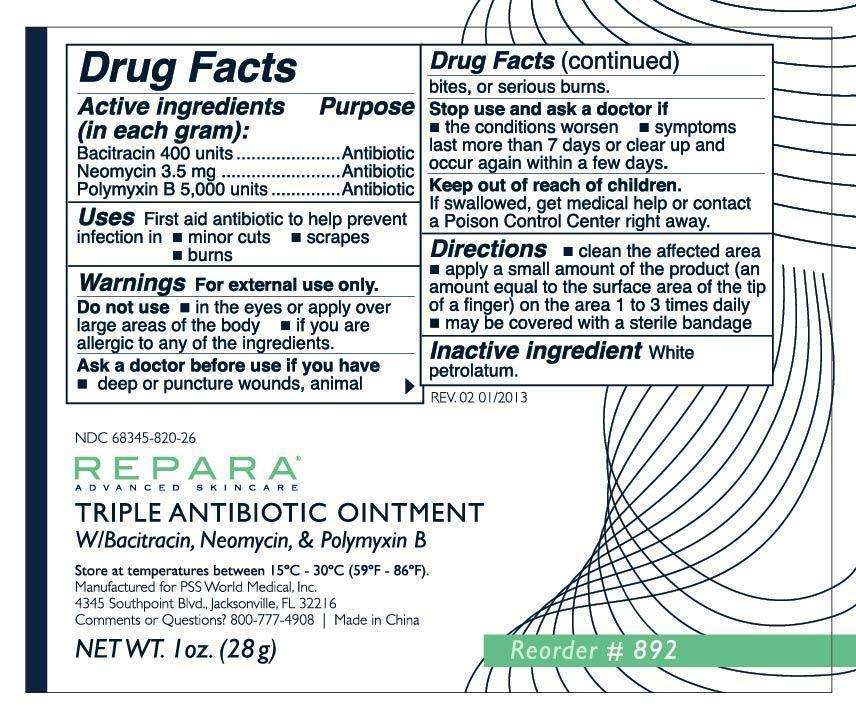
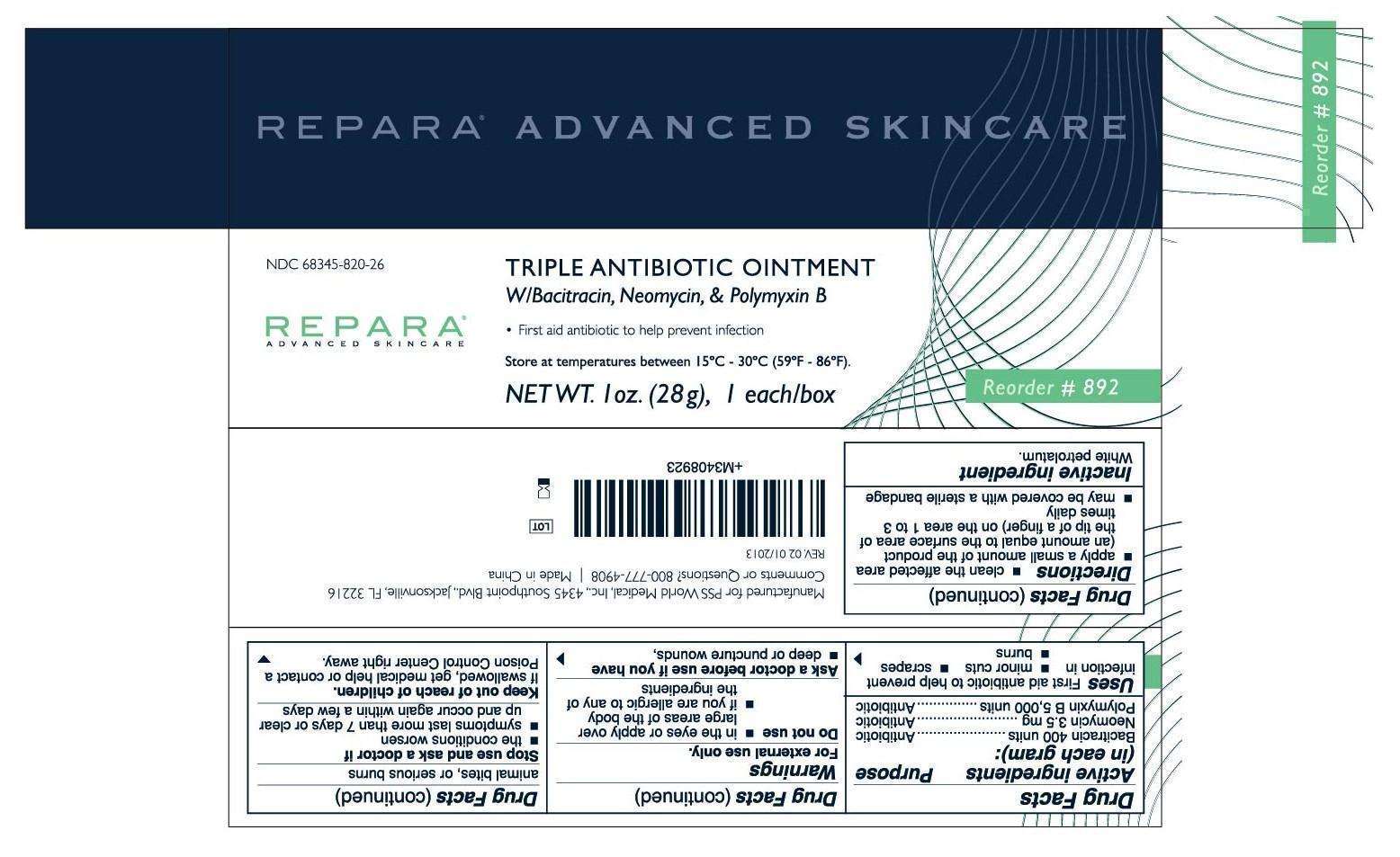
Repara Triple Antibiotic
PSS World Medical, Inc.
Zhejiang Medicare Pharmaceutical Co., Ltd.
REPARA (Triple Antibiotic), ointment
FULL PRESCRIBING INFORMATION
Bacitracin 400 units
Neomycin 3.5 mg
Polymyxin B 5,000 units
Antibiotic
White petrolatum
If swallowed, get medical help or contact a Poison Control Center right away.
First aid antibiotic to help prevent infection in
- minor cuts
- scrapes
- burns
FOR EXTERNAL USE ONLY.
Do not use
• in the eyes or apply over large areas of the body
• if you are allergic to any of the ingredients
Ask a doctor before use if you have
• deep or puncture wounds, animal bites or serious burns
Stop use and ask a doctor if
• the condition worsen
• symptons last more than 7 days or clear up and occur again within a few days
- clean the affected area
- apply a small amount of the product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
- may be covered with a sterile bandage
800-777-4908
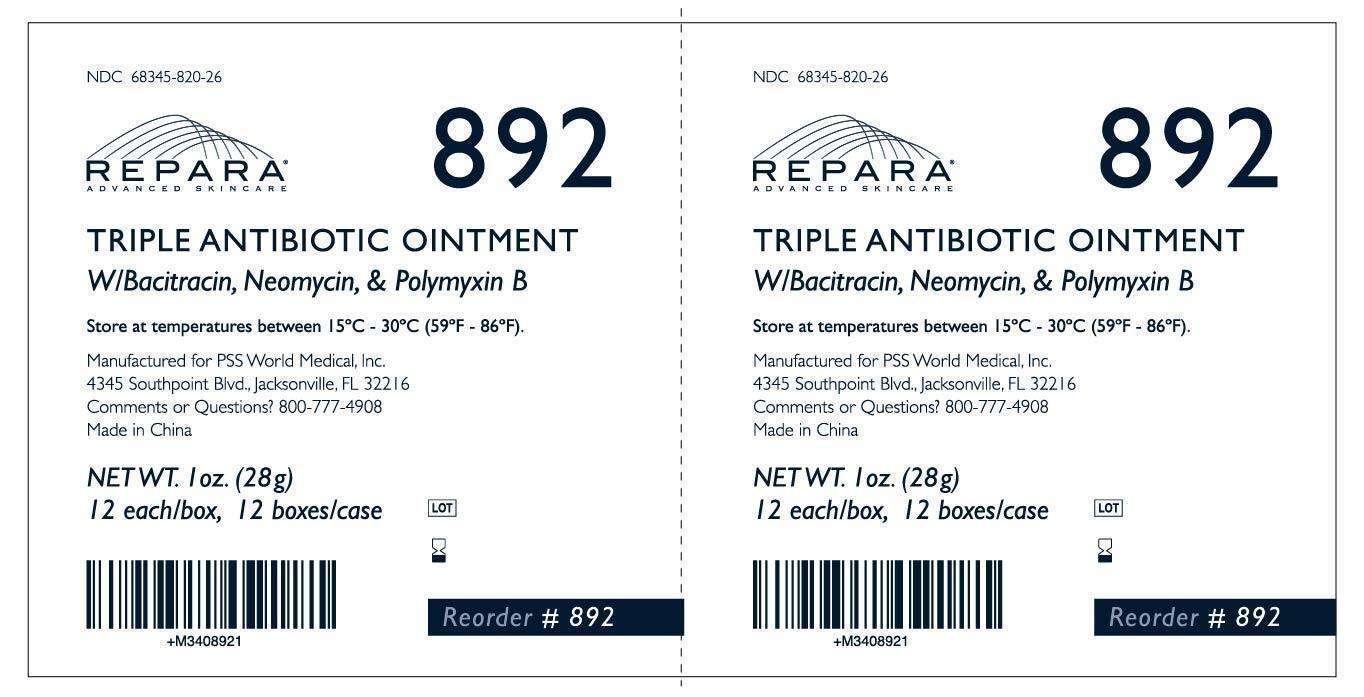
Manufactured for PSS World Medical, Inc.
4345 Southpoint Blvd., Jacksonville, FL 32216
Made in China
Store at temperature between 15°C - 30°C (59°F- 86°F).
Repara® Advanced Skincare
NDC 68345-820-26
Triple Antibiotic Ointment Bacitracin•Neomycin•Polymyxin B
NET WT. 28g. Reorder #892
892_Each
892_EachBox
892_Box

892_Case
Repara Triple AntibioticBACITRACIN, NEOMYCIN and POLYMYXIN B OINTMENT
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||