REDNESS SOLUTIONS MAKEUP
FULL PRESCRIBING INFORMATION
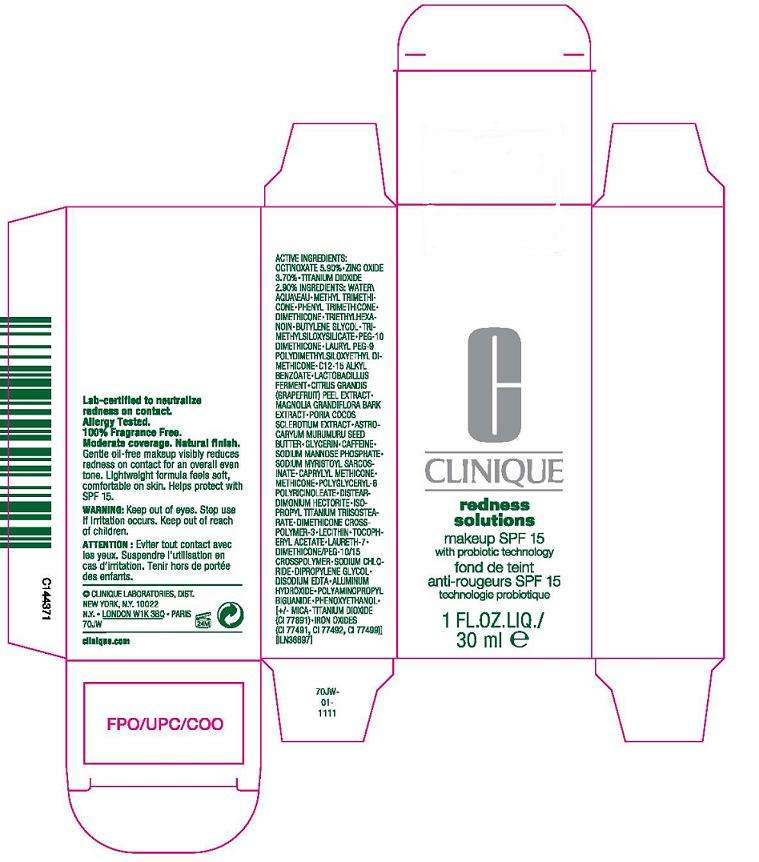
Active ingredient
active ingredients: octinoxate 5.90% [] titanium dioxide 3.70% [] zinc oxide 2.90%
inactive ingredients: water [] methyl trimethicone [] phenyl trimethicone [] dimethicone [] triethylhexanoin [] butylene glycol [] trimethylsiloxysilicate [] peg-10 dimethicone [] lauryl peg-9 polydimethylsiloxyethyl dimethicone [] aluminum hydroxide [] c12-15 alkyl benzoate [] lactobacillus ferment [] citrus grandis (grapefruit) peel extract [] magnolia grandiflora bark extract [] poria cocos sclerotium extract [] astrocaryum murumuru seed butter [] glycerin [] caffeine [] sodium myristoyl sarcosinate [] caprylyl methicone [] methicone [] polyglyceryl-6 polyricinoleate [] disteardimonium hectorite [] acetyl glucosamine [] isopropyl titanium triisostearate [] dimethicone crosspolymer-3 [] lecithin [] tocopheryl acetate [] laureth-7 [] dimethicone/peg-10/15 crosspolymer [] sodium chloride [] dipropylene glycol [] disodium edta [] polyaminopropyl biguanide [] phenoxyethanol [] [+/- mica [] titanium dioxide (ci 77891) [] iron oxides (ci 77491, ci 77492, ci 77499)]
warning:
keep out of eyes. discontinue use if irritation occurs. keep out of reach of children.
principal display panel:
CLINIQUE
REDNESS
SOLUTIONS
MAKE UP SPF 15
with probiotic technology
1 fl oz liq/ 30 ml
clinique laboratories, dist
new york, ny 10022
REDNESS SOLUTIONS MAKEUPOCTINOXATE, TITANIUM DIOXIDE, ZINC OXIDE LOTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||