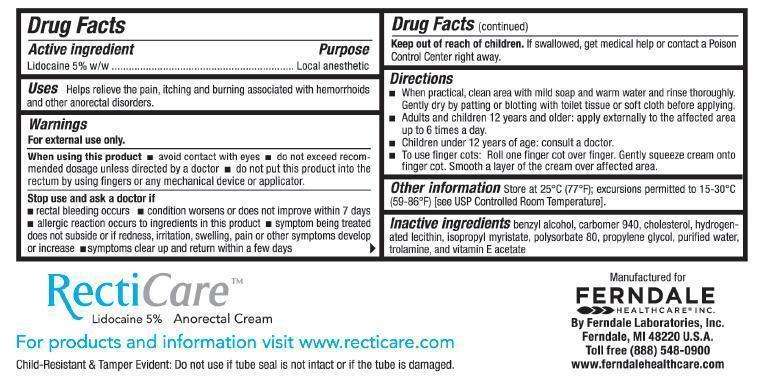
RECTICARE
RECTICARE Lidocaine 5% Anorectal Cream
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- RECTICARE Uses
- Warnings
- Directions
- Other Information
- Inactive Ingredients
- Package Label
FULL PRESCRIBING INFORMATION
Active ingredient
Lidocaine 5% w/w
Purpose
Local anesthetic
RECTICARE Uses
Helps relieve the pain, itching, and burning associated with hemorrhoids and other anorectal disorders.
Warnings
For external use only.
When using this product
- avoid contact with eyes
- do not exceed recommended dosage unless directed by a doctor
- do not put this product into the rectum by using fingers or any mechanical device or applicator
Stop use and ask a doctor if
- rectal bleeding occurs
- condition worsens or does not improve within 7 days
- allergic reaction occurs to ingredients in this product
- symptom being treated does not subside or if redness, irritation, swelling, pain or other symptoms develop or increase
- symptoms clear up and return within a few days
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- When practical, clean area with mild soap and warm water and rinse thoroughly. Gently dry by patting or blotting with toilet tissue or soft cloth before applying.
- Adults and children 12 years and older: apply externally to the affected area up to 6 times a day.
- Children under 12 years of age: consult a doctor.
- To use finger cots: Roll one finger cot over finger. Gently squeeze cream onto finger cot. Smooth a layer of the cream over affected area.
Other Information
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F) [see USP Controlled Room Temperature].
Inactive Ingredients
benzyl alcohol, carbomer 940, cholesterol, hydrogenated lecithin, isopropyl myristate, polysorbate 80, propylene glycol, purified water, trolamine, and vitamin E acetate
Package Label
Manufactured for Ferndale Healthcare® Inc.
By Ferndale Laboratories, Inc.
Ferndale, MI 48220 U.S.A.
Toll free (888) 548-0900
www.ferndalehealthcare.com
30 grams (NDC 0496-0892-30)
Back Carton Panel
Front Carton Panels

RECTICARElidocaine CREAM
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||