Medline Industries, Inc.
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Active ingredient
Dimethicone 3.2%
Purpose
Skin Protectant
Readybath TPC Uses
- temporarily protects and helps relieve chapped or cracked skin
- helps prevent perineal dermatitis
- protects minor skin irritation associated with perineal dermatitis
Warnings
For external use only
Do not use on
- deep or puncture wounds
- animal bites
- serious burns
When using this product
- avoid direct contact with eyes
- discontinue if irritation or redness develops
Stop use and ask a doctor if
- condition worsens
- symptoms last more than 7 days or clear up and occur again within a few days
Keep out of reach of children
If swallowed, get medical help or consult a Poison Control Center right away.
Directions
- Open package as designated
- Remove cloths and thoroughly cleanse soiled area
- Apply as needed
- Dispose of cloth in toilet
Readybath TPC Other information
Store at room temperature.
Inactive ingredients
Principal Display Panel
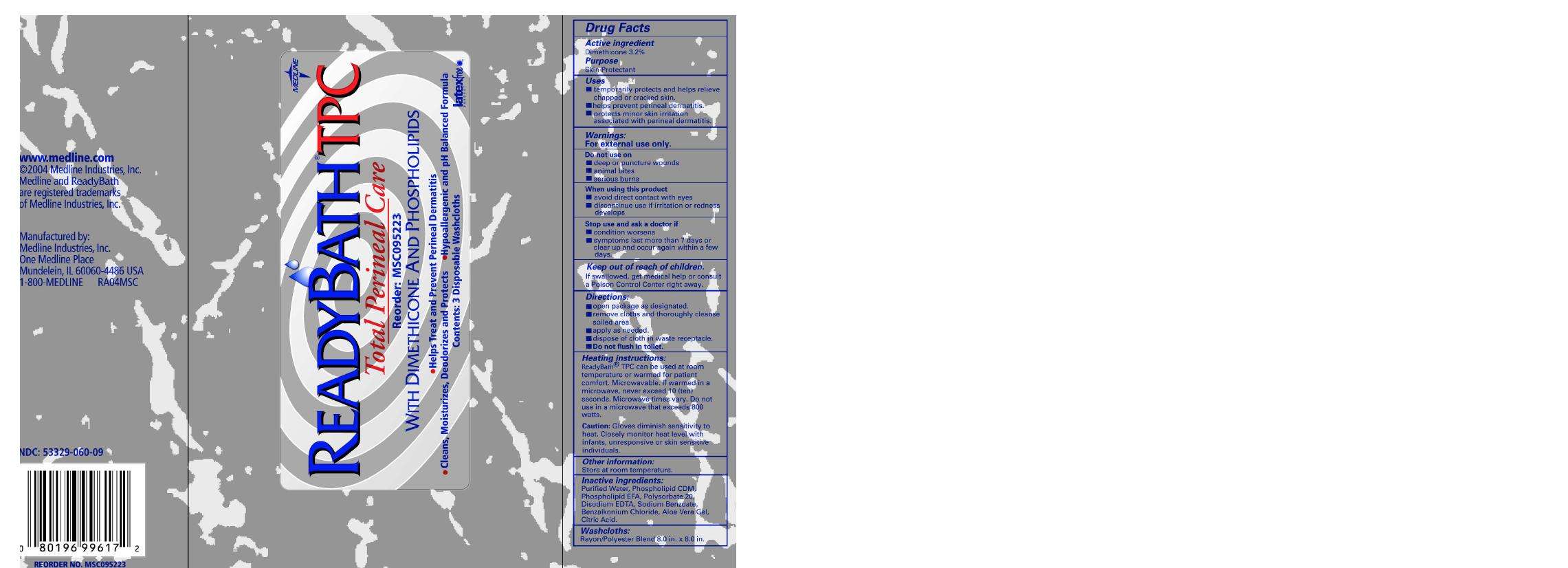
Readybath TPC
Dimethicone CLOTH
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:53329-060 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
DIMETHICONE |
|
0.032 mL
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:53329-060-09 |
3 in 1 PACKAGE |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part347 |
2007-01-01 |
|
|
Readybath TPC
Dimethicone CLOTH
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:53329-061 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
DIMETHICONE |
|
0.032 mL
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:53329-061-09 |
8 in 1 PACKAGE |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part347 |
2007-01-01 |
|
|
Readycleanse
Dimethicone CLOTH
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:53329-062 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
DIMETHICONE |
|
0.032 1
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:53329-062-09 |
24 in 1 PACKAGE |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part347 |
2007-01-01 |
|
|
Aloetouch
Dimethicone CLOTH
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:53329-063 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
DIMETHICONE |
|
0.032 mL
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:53329-063-09 |
48 in 1 PACKAGE |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part347 |
2007-01-01 |
|
|
Aloetouch
Dimethicone CLOTH
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:53329-065 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
DIMETHICONE |
|
0.032 mL
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:53329-065-57 |
60 in 1 PACKAGE |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part347 |
2007-01-01 |
|
|
Readyflush XLarge
Dimethicone CLOTH
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:53329-066 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
DIMETHICONE |
|
0.032 mL
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:53329-066-58 |
24 in 1 PACKAGE |
|
|
|
2 |
NDC:53329-066-62 |
60 in 1 PACKAGE |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part347 |
2007-01-01 |
|
|
![]()