Purpose
Johnson & Johnson Consumer Products Company, Division of Johnson & Johnson Consumer Companies, Inc.
PURPOSE®
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredients
- Purpose Uses
- Warnings
- Directions
- Inactive ingredients
- Other Information
- Questions?
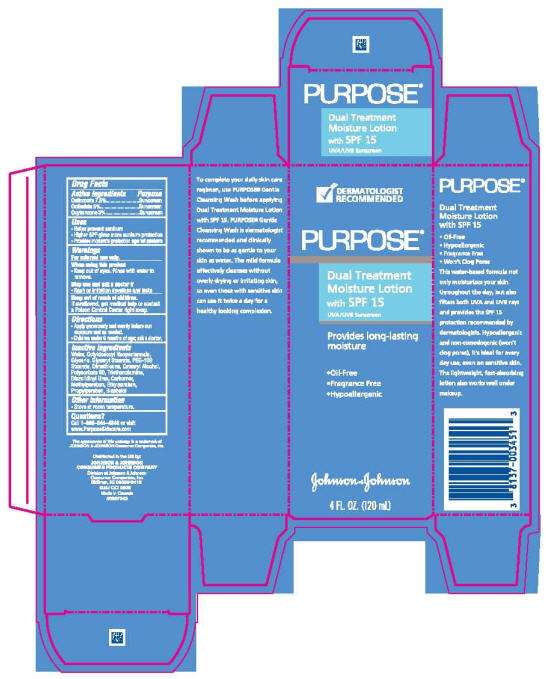
- PRINCIPAL DISPLAY PANEL - 120 mL Carton
FULL PRESCRIBING INFORMATION
Dual Treatment Moisture Lotion SPF 15
Drug Facts
Active ingredients
| Active ingredients | Purpose |
| Octinoxate 7.5%.............................................................................................. | Sunscreen |
| Octisalate 5%................................................................................................. | Sunscreen |
| Oxybenzone 3%................................................................................................. | Sunscreen |
Purpose Uses
- Helps prevent sunburn
- Higher SPF gives more sunburn protection
- Provides moderate protection against sunburn
Warnings
For external use only.
When using this product
- Keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if
- Rash or irritation develops and lasts.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Apply generously and evenly before sun exposure and as needed.
- Children under 6 months of age; ask a doctor.
Inactive ingredients
Water, Octyldodecyl Neopentanoate, Glycerin, Glyceryl Stearate, PEG-100 Stearate, Dimethicone, Cetearyl Alcohol, Polysorbate 60, Triethanolamine, Diazolidinyl Urea, Carbomer, Methylparaben, Ethylparaben, Propylparaben, Bisabolol
Other Information
- Store at room temperature.
Questions?
Call 1-866-344-4848 or visit www.PurposeSkincare.com
PRINCIPAL DISPLAY PANEL - 120 mL Carton
DERMATOLOGIST
RECOMMENDED
PURPOSE®
Dual Treatment
Moisture Lotion
with SPF 15
UVA/UVB Sunscreen
Provides long-lasting
moisture
- Oil-Free
- Fragrance Free
- Hypoallergenic
Johnson & Johnson
4 FL. OZ. (120 mL)
PurposeOctinoxate, Octisalate and Oxybenzone LOTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||