Purell Instant Ultra
PURELL Instant Hand Sanitizer Ultra
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- Use
- Warnings
- Keep Out of Reach of Children
- Directions
- Purell Instant Ultra Other information
- Inactive ingredients
- Package/Label Principal Display Panel
FULL PRESCRIBING INFORMATION
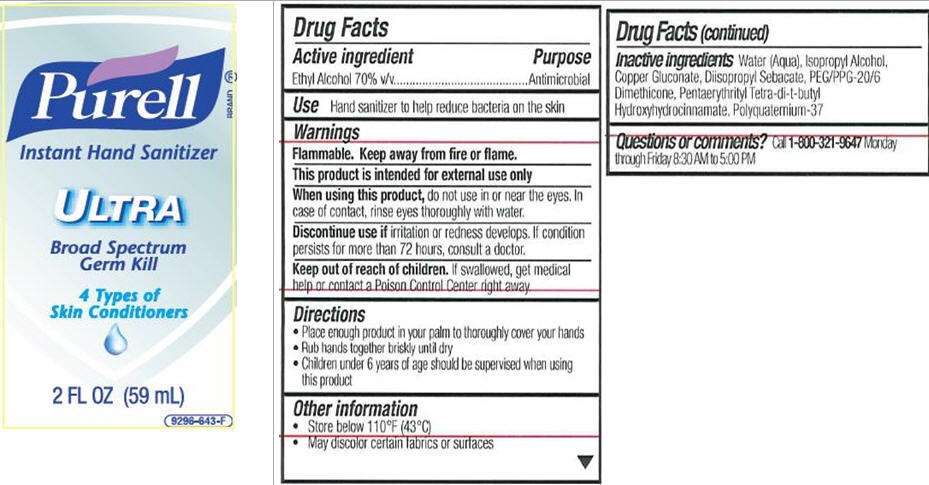
Active ingredient
Ethyl alcohol 70% v/v
Purpose
Antimicrobial
Use
- Hand sanitizer to help reduce bacteria on the skin
Warnings
Flammable. Keep away from fire or flame.
This product is intended for external use only
do not use in or near the eyes. In case of contact, rinse eyes thoroughly with water.
irritation or redness develops. If condition persists for more than 72 hours, consult a doctor
Keep Out of Reach of Children
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Place enough product in your palm to thoroughly cover your hands
- Rub hands together briskly until dry
- Children under 6 years of age should be supervised when using PURELL
Purell Instant Ultra Other information
- Store below 110°F (43°C)
- May discolor certain fabrics or surfaces
- Call 1-800-321-0647 Monday through Friday 8:30 AM to 5:00 PM
Inactive ingredients
Water (Aqua), Isopropyl Alcohol, Copper Gluconate, Diisoprpyl Sebacate, PEG/PPG-20/6 Dimethicone, Pentaerythritl Tetra-di-t-butyl Hydroxyhydrocinnamate, Polyquaternium-37
Package/Label Principal Display Panel
PURELL
Instant Hand Sanitizer
ULTRA
Broad Spectrum Germ Kill
4 Types of Skin Conditioners
2 FL OZ (59 mL)
Bottle Label
Purell Instant Ultraalcohol GEL
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!