Profender
Bayer HealthCare LLC Animal Health Division
Profender (emodepside/praziquantel)Topical Solution
FULL PRESCRIBING INFORMATION: CONTENTS*
- PROFENDER DESCRIPTION:
- INDICATIONS:
- PROFENDER DOSAGE AND ADMINISTRATION:
- HUMAN WARNINGS:
- PRECAUTIONS:
- PROFENDER ADVERSE REACTIONS:
- EFFECTIVENESS:
- ANIMAL SAFETY:
- STORAGE INFORMATION:
- HOW SUPPLIED:
- PRINCIPAL DISPLAY PANEL - Small Cats and Kittens (2.2 - 5.5 lbs), 20 (0.35 ml) tubes:
- PRINCIPAL DISPLAY PANEL - Small Cats and Kittens (2.2 - 5.5 lbs), 40 (0.35 ml) tubes:
- PRINCIPAL DISPLAY PANEL - Medium Cats and Kittens (> 5.5 - 11 lbs.), 20 (0.70 ml) tubes:
- PRINCIPAL DISPLAY PANEL - Medium Cats and Kittens (> 5.5 - 11 lbs.), 40 (0.70 ml) tubes:
- PRINCIPAL DISPLAY PANEL - Large Cats and Kittens (> 11 - 17.6 lbs.), 20 (1.12 ml) tubes:
- PRINCIPAL DISPLAY PANEL - Large Cats and Kittens (> 11 - 17.6 lbs.), 40 (1.12 ml) tubes:
FULL PRESCRIBING INFORMATION
CAUTION: Federal law (U.S.A.) restricts this drug to use by or on the order of a licensed veterinarian.
Topical Solution for the treatment and control of hookworm, roundworm and tapeworm infections in cats and kittens that are at least 8 weeks of age and weigh at least 2.2 lbs (1 kg).
DESCRIPTION:
PROFENDER [1.98% emodepside/7.94% praziquantel] Topical Solution is a clear yellow ready to use solution packaged in single unit dosing applicator tubes for topical (dermal) treatment of cats 8 weeks of age and older and weighing at least 2.2 lbs (1 kg). The formulation and dosage schedule is designed to provide a minimum of 1.36 mg/lb (3 mg/kg) emodepside and 5.45 mg/lb (12 mg/kg) praziquantel based on body weight. Emodepside, a semi-synthetic molecule, is a cyclic depsipeptide. The chemical name is Cyclo [D-2-hydroxypropanoyl-N-methyl-L-leucyl-3-[4-(4-morpholinyl)phenyl]-D-2-hydroxypropanoyl-N-methyl-L-leucyl-D-2-hydroxypropanoyl-N-methyl-L-leucyl-3-[4-(4-morpholinyl)phenyl]-D-2-hydroxypropanoyl-N-methyl-L-leucyl]. Praziquantel is an isoquinoline cestocide. The chemical name is 2-Cyclohexylcarbonyl- 1,2,3,6,7,11b-hexahydro-4H-pyrazine-2,1-a-isoquinoline- 4-one.
INDICATIONS:
PROFENDER Topical Solution is indicated for the treatment and control of hookworm infections caused by Ancylostoma tubaeforme (adults, immature adults, and fourth stage larvae), roundworm infections caused by Toxocara cati (adults and fourth stage larvae), and tapeworm infections caused by Dipylidium caninum (adults) and Taenia taeniaeformis (adults) in cats.
DOSAGE AND ADMINISTRATION:
The recommended minimum dose is 1.36 mg/lb (3 mg/kg) emodepside + 5.45 mg/lb (12 mg/kg) praziquantel as a single topical dose. A single treatment is effective and a second treatment should not be necessary. If re-infection occurs, the product can be re-applied after 30 days.
1. Select the package that correctly corresponds with the body weight of the cat. (See Table below.)
|
Cat Weight* |
Profender Topical Solution |
Volume (mL) |
Emodepside (mg) |
Praziquantel (mg) |
|
2.2-5.5 lbs. |
Small |
0.35 |
7.5 |
30.0 |
|
>5.5-11 lbs. |
Medium |
0.70 |
15.0 |
60.1 |
|
>11-17.6 lbs. |
Large |
1.12 |
24.0 |
96.1 |
| * Cats over 17.6 lbs should be treated with the appropriate combination of tubes. | ||||
2. Remove one unit dose tube from the package.
3. While holding the tube in an upright position, remove the cap from the tube.
4. Turn the cap over and place the other end of cap onto the tip of the tube.
5. Twist the cap to break the seal and then remove cap from the tube.

6. Part the hair on the back of the cat’s neck at the base of the head, until the skin is visible.
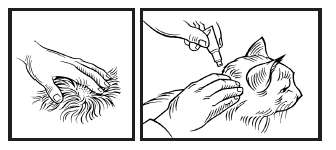
7. To ensure the entire contents of the tube are administered, place the tip of the tube on the skin and squeeze the entire contents directly onto the skin. Lift tube away from the skin before releasing pressure on the tube.
Do not apply to broken skin or if hair coat is wet.
Do not get this product in the cat’s mouth or eyes or allow the cat to lick the application site for one hour. Oral exposure can cause salivation and vomiting. Treatment at the base of the head will minimize the opportunity for ingestion while grooming. In households with multiple pets, keep animals separated to prevent licking of the application site.
Stiff hair, a damp appearance of the hair, or a slight powdery residue may be observed at the treatment site. These effects are temporary and do not affect the safety or effectiveness of the product.
HUMAN WARNINGS:
Not for human use. Keep out of reach of children.
To prevent accidental ingestion of the product, children should not come in contact with the application site for twenty-four (24) hours while the product is being absorbed. Pregnant women, or women who may become pregnant, should avoid direct contact with, or wear disposable gloves when applying, this product. Studies performed in rats and rabbits suggest that emodepside may interfere with fetal development in those species.
PROFENDER Topical Solution may be irritating to skin and eyes. Reactions such as facial, tongue and hand swelling have been reported in humans in rare instances. Avoid contact with the application area while it is wet and wash hands thoroughly with soap and warm water after handling. People with known hypersensitivity to butylhydroxyanisole, emodepside or praziquantel should administer the product with caution. If the product accidentally gets into eyes, flush thoroughly with water. May be harmful if swallowed. In case of accidental ingestion or if skin or eye irritation occurs, call a poison control center or physician for treatment advice.
The Material Safety Data Sheet (MSDS) provides additional occupational safety information. For customer service or to obtain product information, including the MSDS, call 1-800-633-3796. For medical emergencies or to report an adverse reaction, call 1-800-422-9874.
PRECAUTIONS:
Safe use of this product has not been evaluated in cats less than 8 weeks of age or weighing less than 2.2 lbs (1 kg), in cats used for breeding, during pregnancy or in lactating queens. The effectiveness of this product when used before bathing has not been evaluated.
Use with caution in sick or debilitated cats. Oral ingestion or exposure should be avoided. Use with caution in heartworm positive cats. The cats enrolled in the field study were heartworm antigen and antibody negative prior to entering the study. In a laboratory study, cats artificially infected with adult heartworms and treated with PROFENDER Topical Solution had fewer worms recovered than the placebo control group. (See ANIMAL SAFETY. )
ADVERSE REACTIONS:
Field study: In a controlled, double-masked field safety study, owners administered PROFENDER Topical Solution to 606 cats. Adverse reactions reported by the cat owners included licking/ excessive grooming in 18 cats (3.0%), scratching treatment site in 15 cats (2.5%), salivation in 10 cats (1.7%), lethargy in 10 cats (1.7%), alopecia in 8 cats (1.3%), agitation/nervousness in 7 cats (1.2%), vomiting in 6 cats (1.0%), diarrhea in 3 cats (0.5%), eye irritation in 3 cats (0.5%), respiratory irritation in 1 cat (0.2%) and shaking/tremors in 1 cat (0.2%). All adverse reactions were self-limiting.
Laboratory effectiveness studies: One cat died 10 days after receiving PROFENDER Topical Solution. The necropsy showed chronic active cholangiohepatitis. While the use of the drug did not appear to be the direct cause of death, treatment with the drug cannot be ruled out as a contributing factor (See PRECAUTIONS ). One cat treated with a vehicle placebo (formulation minus the active ingredients) showed salivation, gagging, lethargy and a swollen tongue.
Foreign Market Experience: The following adverse events were reported voluntarily during post-approval use of the product in foreign markets: application site reaction (hair loss, dermatitis, pyoderma, edema, and erythema), salivation, pruritus, lethargy, vomiting, diarrhea, dehydration, ataxia, loss of appetite, facial swelling, rear leg paresis, seizures, hyperesthesia, twitching, and death.
Post-Approval Experience: The following adverse events are based on post-approval adverse drug experience reporting. Not all adverse events are reported to FDA CVM. It is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using this data. The following adverse events are listed in decreasing order of reporting frequency in cats: Application site reaction (hair loss, dermatitis, pyoderma, edema, and erythema), hypersalivation, lethargy/depression, vomiting, ataxia, anorexia, trembling/twitching, diarrhea, mydriasis, fever, hyperactivity/nervousness. In some cases, death has been reported as an outcome of the adverse events listed. For a complete listing of adverse reactions for Profender Topical Solution reported to the CVM see: http://www.fda.gov/ADEreports.
This listing includes Adverse Events reported to CVM for products, such as Profender, that contain the combined active ingredients emodepside and praziquantel. Listings by active ingredient may represent more than one brand name.
To report suspected adverse events and/or obtain a copy of the MSDS or for technical assistance, call Bayer Animal Health at 1-800-633-3796.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at http://www.fda.gov/AnimalVeterinary/SafetyHealth.
EFFECTIVENESS:
In a total of 13 controlled laboratory studies to establish effectiveness, 149 cats were treated with PROFENDER Topical Solution. In the field study conducted at 13 veterinary clinics/hospitals, 837 purebred or crossbred cats from single and multicat households were enrolled to evaluate safety and effectiveness under field conditions of use. Of those, 606 received a single treatment with PROFENDER Topical Solution. Cats ranged in age between 2 months and 17 years and weighed between 0.8 lbs (0.36 kg) and 21 lbs (9.62 kg). Data from these studies demonstrated PROFENDER Topical Solution is safe and effective for the treatment and control of hookworm infections caused by Ancylostoma tubaeforme (adults, immature adults, and fourth stage larvae), roundworm infections caused by Toxocara cati (adults and fourth stage larvae), and tapeworm infections caused by Dipylidium caninum (adults) and Taenia taeniaeformis (adults).
ANIMAL SAFETY:
In a field study, PROFENDER Topical Solution was used in cats receiving other frequently used products including: analgesics, anti-fungals, nonsteroidal anti-inflammatories, anthelmintics, antimicrobials, flea and tick products, sedatives, anesthetics, cardiac medications, anxiolytics, hormonal treatments, steroids, otic and ophthalmic preparations, and vaccines.
Dose Tolerance Study in Cats: PROFENDER Topical Solution was applied topically one time to young cats at 10X the recommended label use rate. Two cats salivated. Another cat exhibited tremors and lethargy. These signs were self-limiting.
Oral Safety Studies in Cats: PROFENDER Topical Solution was administered orally at the recommended topical dose to young adult cats. The cats exhibited salivation, vomiting, tremors, abnormal gait, abnormal respiration and weight loss. These signs were self-limiting.
General Safety Study in Kittens: PROFENDER Topical Solution was topically applied at 0X (vehicle control), 1X, 3X and 5X the maximum dose to 48 healthy 8-week-old kittens every two weeks for six doses. One 5X kitten experienced salivation and tremors and another 5X kitten experienced salivation on the day of dosing. A third 5X kitten experienced tremors the day after dosing. Three cats vomited within 24 hours of dosing, one each in vehicle control, 3X and 5X groups.
Safety Study in Heartworm Positive Cats: Cats artificially infected with adult heartworms harvested from dogs were treated topically with PROFENDER Topical Solution at 0X, 1X or 5X the recommended dose once a month for three treatments. Clinical signs included salivation (one 1X and three 5X cats), labored breathing (all groups) and lethargy (one 5X cat). At the study conclusion, the 1X and 5X cats had fewer live heartworms recovered than the 0X group.
STORAGE INFORMATION:
Store at or below 77ºF (25ºC).
Protect from freezing.
HOW SUPPLIED:
Code Number Applications per Package
82482521 20 - 0.35 mL tubes (5 blisters of 4 tubes)
03615026 40 - 0.35 mL tubes (10 blisters of 4 tubes)
82482572 20 - 0.70 mL tubes (5 blisters of 4 tubes)
03615034 40 - 0.70 mL tubes (10 blisters of 4 tubes)
82482580 20 - 1.12 mL tubes (5 blisters of 4 tubes)
82482602 40 - 1.12 mL tubes (10 blisters of 4 tubes)
Profender is protected by the following U.S. Patents:
5514773 and other patents pending.
Made in Germany
NADA 141-275, Approved by FDA
© 2013 Bayer HealthCare LLC
Bayer, the Bayer Cross and Profender are registered trademarks of Bayer.
82482521/03615026/82482572/03615034/82482580/82482602, R.2
February, 2013
Bayer
Bayer HealthCare LLC
Animal Health Division
P.O. Box 390
Shawnee Mission, Kansas 66201 U.S.A.
PRINCIPAL DISPLAY PANEL - Small Cats and Kittens (2.2 - 5.5 lbs), 20 (0.35 ml) tubes:

PRINCIPAL DISPLAY PANEL - Small Cats and Kittens (2.2 - 5.5 lbs), 40 (0.35 ml) tubes:

PRINCIPAL DISPLAY PANEL - Medium Cats and Kittens (> 5.5 - 11 lbs.), 20 (0.70 ml) tubes:

PRINCIPAL DISPLAY PANEL - Medium Cats and Kittens (> 5.5 - 11 lbs.), 40 (0.70 ml) tubes:

PRINCIPAL DISPLAY PANEL - Large Cats and Kittens (> 11 - 17.6 lbs.), 20 (1.12 ml) tubes:

PRINCIPAL DISPLAY PANEL - Large Cats and Kittens (> 11 - 17.6 lbs.), 40 (1.12 ml) tubes:

ProfenderEmodepside/Praziquantel SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
ProfenderEmodepside/Praziquantel SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
ProfenderEmodepside/Praziquantel SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||