ProClearz
ProClearz Anti-Fungal Brush-On Maximum Strength - Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredient
- Purpose
- ProClearz Uses
- Warnings
- Directions
- Other Information
- Inactive ingredients
- Image of Label and Carton
FULL PRESCRIBING INFORMATION
Active Ingredient
Tolnaftate 1%
Purpose
Anti-fungal
ProClearz Uses
- cures most athlete's foot (tinea pedis) and ringworm (tinea corporis)
- helps prevent most athlete's foot from recurring when used daily
- relieves itching, scaling, cracking, burning, and redness
Warnings
For external use only
- do not use on children under 2 years of age unless directed by a doctor
Extremely flammable
- keep away from fire or flame
- avoid contact with the eyes
- if contact occurs rinse thoroughly with water
- irritation occurs or if there is no improvement in 4 weeks
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away
Directions
■ shake well before using ■ wash affected area and dry thoroughly ■ apply a thin layer of the
product over affected area (around the nails and underneath nail tips) twice daily or as directed
by a doctor ■ supervise children in the use of this product ■ for athlete’s foot, pay special
attention to spaces between the toes; wear well fitting, ventilated shoes and change shoes and
socks at least once daily ■ for athlete’s foot and ringworm, use daily for 4 weeks. If condition
persists longer, consult a doctor ■ this product is not effective on the scalp or nails
Other Information
- store at room temperature 15 - 30 degrees C (59 - 86 degrees F)
Inactive ingredients
Acetone, Aloe Vera (Aloe Barbadensis) Gel, Alpha Tocopherol Acetate (Vitamin E), Propylene Glycol, Water
Image of Label and Carton
ProClearzLabelFDA0712copyESG.jpg ProClearz5panelFDA0712v7ESG.jpg
ProClearz5panelFDA0712v7ESG.jpg ProClearzVerticalFDA06122v5ESG.jpg
ProClearzVerticalFDA06122v5ESG.jpg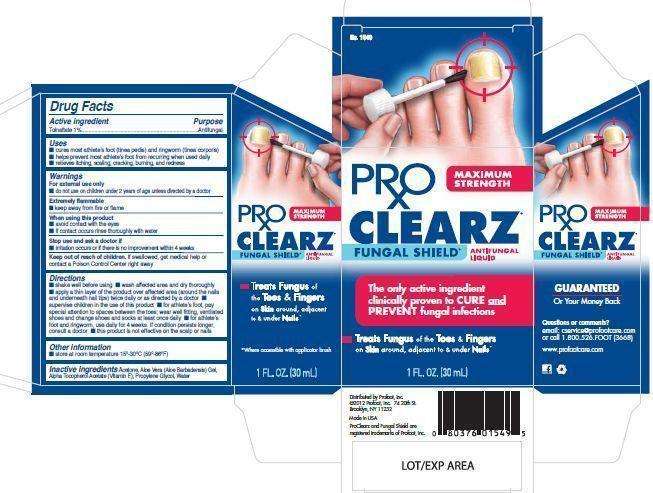
ProClearzTolnaftate LIQUID
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||